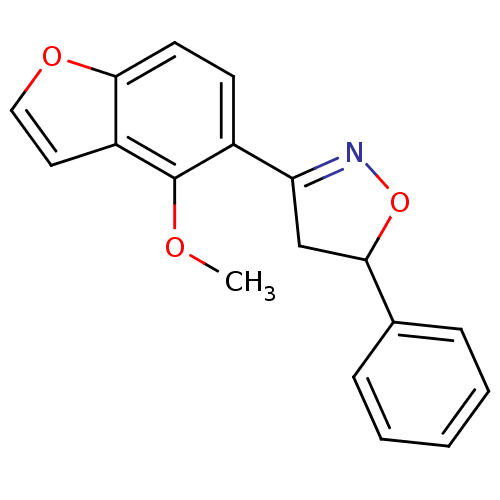
BDBM50182135 3-(4-methoxybenzofuran-5-yl)-5-phenyl-4,5-dihydroisoxazole::CHEMBL208485
SMILES COc1c(ccc2occc12)C1=NOC(C1)c1ccccc1
InChI Key InChIKey=USHIACGOJWXTMQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50182135
Found 3 hits for monomerid = 50182135
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.60E+4nMAssay Description:Inhibition of PTP1B (unknown origin) using p-nitrophenylphosphate as substrate after 10 minMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.27E+5nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair