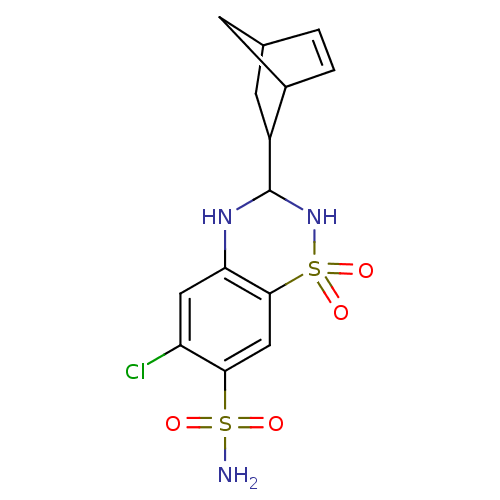
BDBM50192229 3-Bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-benzo[1,2,4]thiadiazine-7-sulfonic acid amide::3-Bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-benzo[1,2,4]thiadiazine-7-sulfonic acid amide(Clothiazide)::3-Bicyclo[2.2.1]hept-5-en-2-yl-7-chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-benzo[1,2,4]thiadiazine-6-sulfonic acid amide::6-chloro-3,4-dihydro-3-(5-norbornen-2-yl)-2H-1,2,4-benzothiazidiazine-7-sulfonamide-1,1-dioxide::CHEMBL61593::CYCLOTHIAZIDE
SMILES NS(=O)(=O)c1cc2c(NC(NS2(=O)=O)C2CC3CC2C=C3)cc1Cl
InChI Key InChIKey=BOCUKUHCLICSIY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50192229
Found 1 hit for monomerid = 50192229
TargetGlutamate receptor 2(Rattus norvegicus)
European Research Centre For Drug Discovery And Development (Natsyndrugs)
Curated by ChEMBL
European Research Centre For Drug Discovery And Development (Natsyndrugs)
Curated by ChEMBL
Affinity DataEC50: 7.60E+3nMAssay Description:Agonist activity at rat flip iGluR2(Q) expressed in Xenopus laevis oocytes by whole cell patch-clamp methodMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)