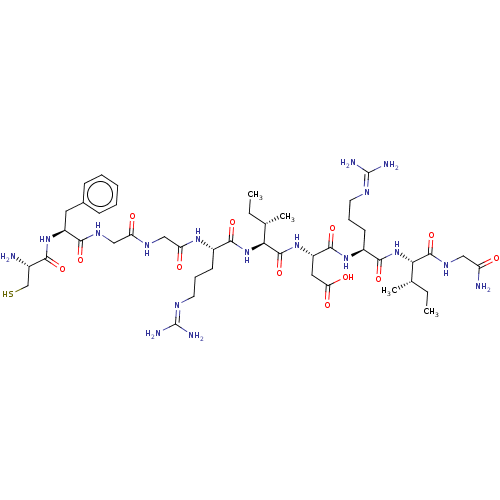
BDBM50228175 CHEMBL265243
SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O
InChI Key InChIKey=ITBHBSGWWGRWKQ-HYROADNPSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50228175
Found 1 hit for monomerid = 50228175
Affinity DataIC50: >100nMAssay Description:Inhibitory activity against guanylate cyclase coupled receptor binding site in rabbit lung by using [125I]-ANP-(103-126)More data for this Ligand-Target Pair