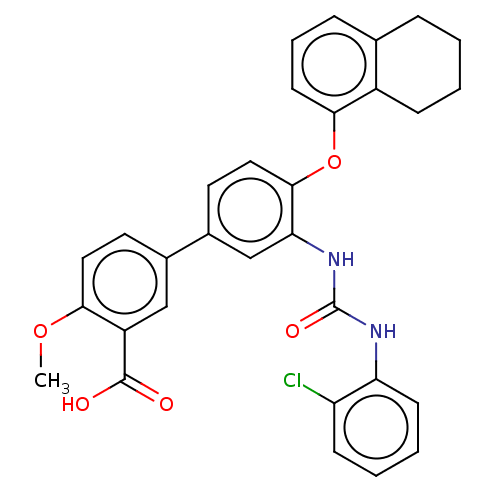
BDBM50234040 CHEMBL4060089::US9765018, Example 114
SMILES COc1ccc(cc1C(O)=O)-c1ccc(Oc2cccc3CCCCc23)c(NC(=O)Nc2ccccc2Cl)c1
InChI Key InChIKey=OLUVZKHXNIKBIS-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50234040
Found 10 hits for monomerid = 50234040
TargetCytochrome P450 2C9(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using BZR as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as inhibition of kynurenine production preincubated with cells followed by IFN-g...More data for this Ligand-Target Pair
TargetCytochrome P450 2B6(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using BFC as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair