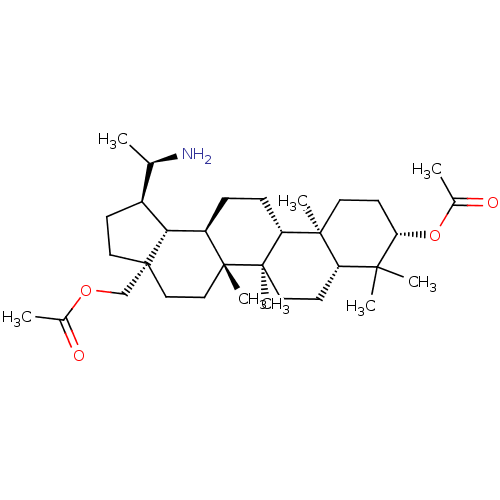
BDBM50234803 CHEMBL4099629
SMILES [H][C@]1(CC[C@]2(COC(C)=O)CC[C@]3(C)[C@]([H])(CC[C@]4([H])[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@]5([H])CC[C@@]34C)[C@@]12[H])[C@@H](C)N
InChI Key InChIKey=MFXQEPNZKMYOJG-VIFUSZNRSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50234803
Found 3 hits for monomerid = 50234803
TargetCholinesterase(Equus caballus (Horse))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 370nMAssay Description:Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 4.50E+3nMAssay Description:Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu...More data for this Ligand-Target Pair