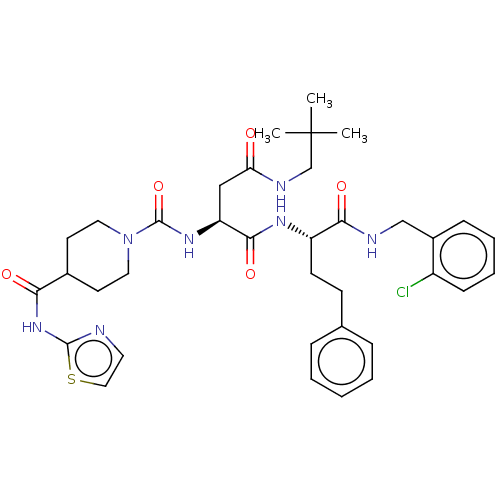
BDBM50234982 CHEMBL4102324
SMILES CC(C)(C)CNC(=O)C[C@H](NC(=O)N1CCC(CC1)C(=O)Nc1nccs1)C(=O)N[C@@H](CCc1ccccc1)C(=O)NCc1ccccc1Cl
InChI Key InChIKey=MFJLZSWPUNUKNF-VMPREFPWSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50234982
Found 2 hits for monomerid = 50234982
TargetA5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436(Homo sapiens (Human))
Zhejiang University
Curated by ChEMBL
Zhejiang University
Curated by ChEMBL
Affinity DataIC50: 0.920nMAssay Description:Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu Leu-Val-Tyr-AMC as substrate preincubated for 15 mins followed by subs...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of chymotrypsin-like activity of human 20S proteasome pretreated for 15 mins followed by Suc-Leu-Leu-Val-Tyr-AMC substrate addition by flu...More data for this Ligand-Target Pair