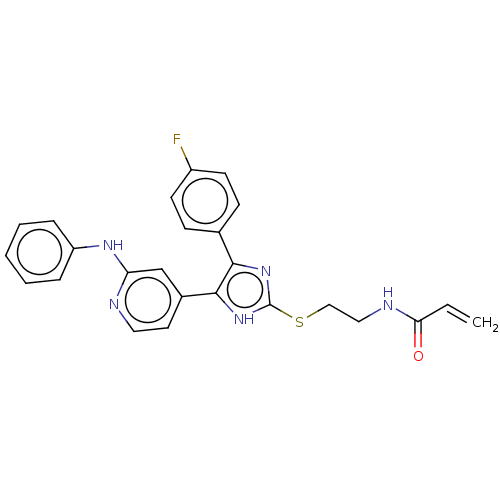
BDBM50238183 CHEMBL4071012
SMILES Fc1ccc(cc1)-c1nc(SCCNC(=O)C=C)[nH]c1-c1ccnc(Nc2ccccc2)c1
InChI Key InChIKey=KIRCCODEAPBPMW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50238183
Found 2 hits for monomerid = 50238183
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenateMore data for this Ligand-Target Pair