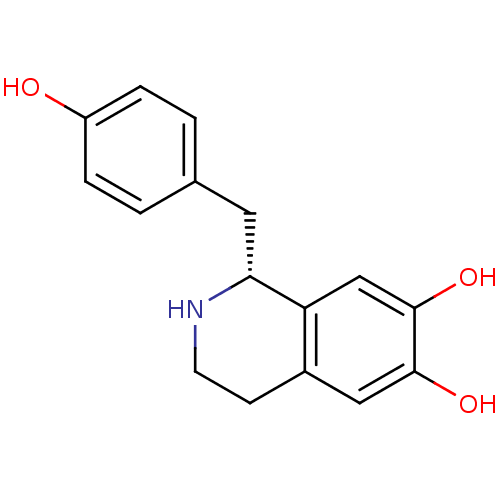
BDBM50242856 (1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquinoline-6,7-diol::(R)-norcoclaurine::CHEMBL501778
SMILES Oc1ccc(C[C@H]2NCCc3cc(O)c(O)cc23)cc1
InChI Key InChIKey=WZRCQWQRFZITDX-CQSZACIVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50242856
Found 3 hits for monomerid = 50242856
Affinity DataKi: 3.10E+3nMAssay Description:Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 4.50E+3nMAssay Description:Agonist activity at beta2 adrenergic receptor in rat L6 cell lysate assessed as increase in intracellular 2-deoxyglucose uptake preincubated for 4 hr...More data for this Ligand-Target Pair