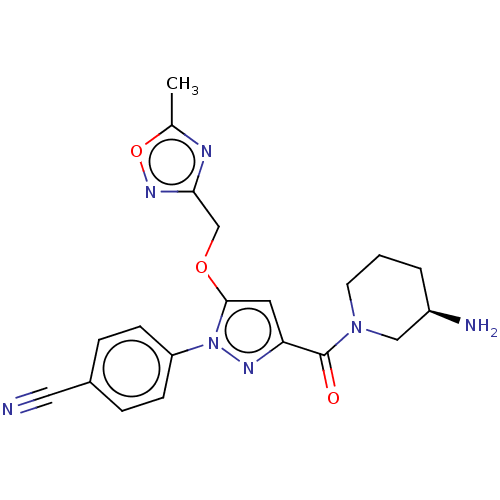
BDBM50242905 CHEMBL4103503
SMILES Cc1nc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCC[C@@H](N)C2)no1
InChI Key InChIKey=BINMIFARNCTJDS-OAHLLOKOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50242905
Found 3 hits for monomerid = 50242905
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of Fibrinogen receptor bindingMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibition of LSD1 in human THP1 cells assessed as upregulation of CD86 levels by ELISAMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataKd: 34nMAssay Description:Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysisMore data for this Ligand-Target Pair