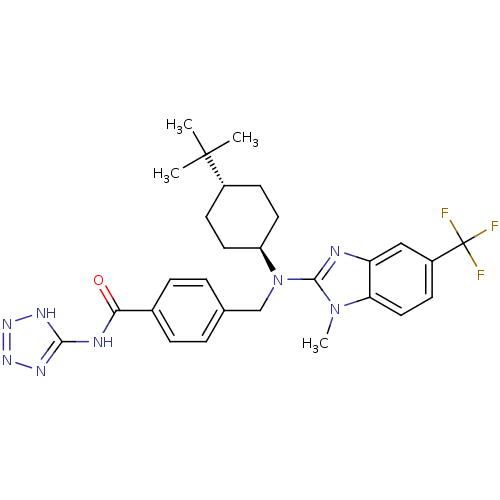
BDBM50244299 CHEMBL480501::trans-4-(((4-tert-butylcyclohexyl)(1-methyl-5-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)amino)methyl)-N-(1H-tetrazol-5-yl)benzamide
SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C
InChI Key InChIKey=WSMJMACKNHBKSD-XUTJKUGGSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50244299
Found 8 hits for monomerid = 50244299
Affinity DataIC50: 62nMAssay Description:Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80E+3nMAssay Description:Binding affinity against human GLP1More data for this Ligand-Target Pair
TargetGastric inhibitory polypeptide(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Binding affinity against human GIPMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Displacement of [125I]glucagon from human GCGR expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of hERG potassium channelMore data for this Ligand-Target Pair