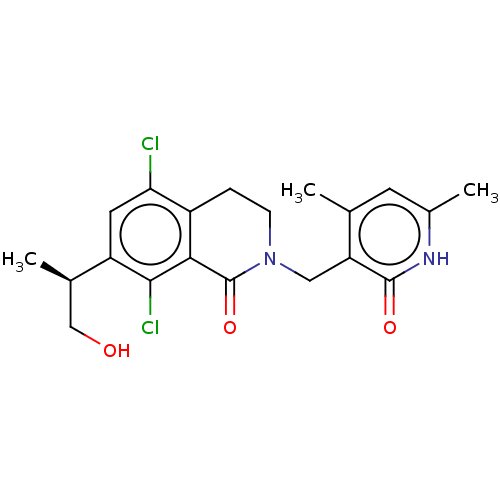
BDBM50246964 CHEMBL4060640::US10570121, Example 18
SMILES C[C@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl
InChI Key InChIKey=GMVWARWJGLYCAS-LLVKDONJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50246964
Found 4 hits for monomerid = 50246964
Affinity DataKi: 115nMAssay Description:A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ...More data for this Ligand-Target Pair
Affinity DataIC50: 995nMAssay Description:A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ...More data for this Ligand-Target Pair
Affinity DataIC50: 129nMAssay Description:Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ...More data for this Ligand-Target Pair