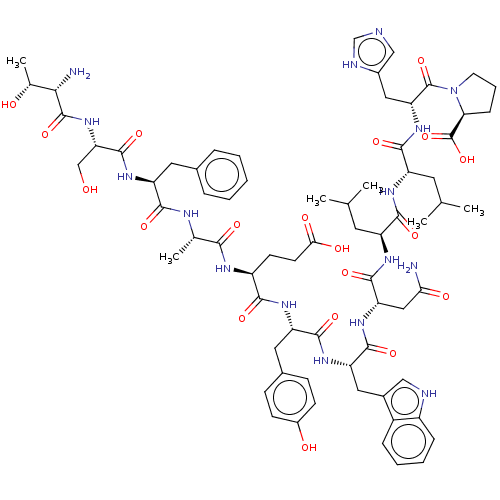
BDBM50261658 CHEMBL4099164
SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(O)=O
InChI Key InChIKey=ZYPYAXPTWKNCLK-LPSCLBFBSA-N
Data 2 Kd
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50261658
Found 2 hits for monomerid = 50261658
Affinity DataKd: 14nMAssay Description:Agonist activity at human beta-2 adrenergic receptor expressed in human H292 cells assessed as accumulation of intracellular cAMP after 1 hr by Alpha...More data for this Ligand-Target Pair
Affinity DataKd: 61nMAssay Description:Cross Resistance (antiviral activity) of the compound with Y188C (Nonnucleoside reverse transcriptase inhibitor) resistant HIV isolate from cytopathi...More data for this Ligand-Target Pair