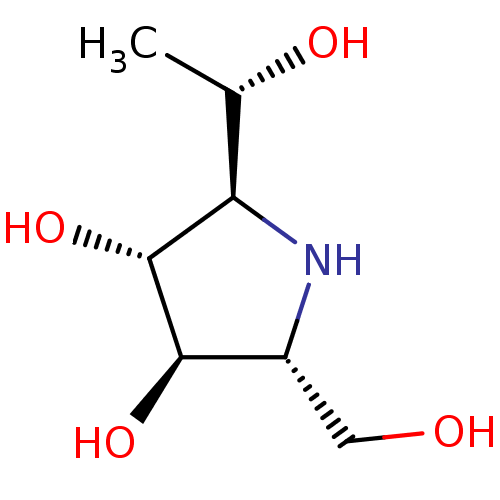
BDBM50279407 2,5-Imino-2,5,7-trideoxy-D-glycero-D-manno-heptitol::CHEMBL488157
SMILES C[C@H](O)[C@H]1N[C@H](CO)[C@@H](O)[C@@H]1O
InChI Key InChIKey=GBQAQDYHLUTAGE-CQOGJGKDSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50279407
Found 4 hits for monomerid = 50279407
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of rat epididymis beta-mannosidase assessed as p-nitrophenol release after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of Caldocellum saccharolyticum beta-glucosidase assessed as D-glucose release after 30 mins by Glucose B-testMore data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
Hokuriku University
Curated by ChEMBL
Hokuriku University
Curated by ChEMBL
Affinity DataIC50: 8.50E+4nMAssay Description:Inhibition of yeast alpha-glucosidase assessed as D-glucose release after 30 mins by Glucose B-testMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of bovine liver beta-galactosidase assessed as p-nitrophenol release after 30 mins by spectrophotometryMore data for this Ligand-Target Pair