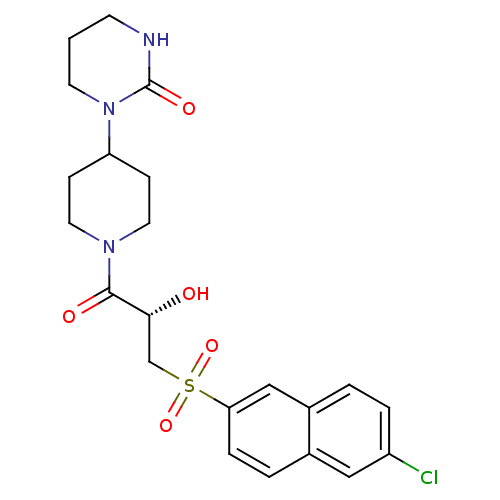
BDBM50317098 1-(1-{(2S)-3-[(6-chloronaphthalen-2-yl)sulfonyl]-2-hydroxypropanoyl}piperidin-4-yl)tetrahydropyrimidin-2(1H)-one::CHEMBL1095032::TAK-442
SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCNC1=O
InChI Key InChIKey=GEHAEMCVKDPMKO-HXUWFJFHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50317098
Found 8 hits for monomerid = 50317098
Affinity DataKi: 1.80nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human thrombin after 10 mins by chromogenic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human factor 9a after 10 mins by chromogenic assayMore data for this Ligand-Target Pair
Affinity DataIC50: >6.00E+4nMAssay Description:Inhibition of human plasmin after 5 mins by chromogenic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human factor 10a using S2222 as substrate after 10 mins by chromogenic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+4nMAssay Description:Inhibition of human tissue plasminogen activator after 5 mins by chromogenic assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)