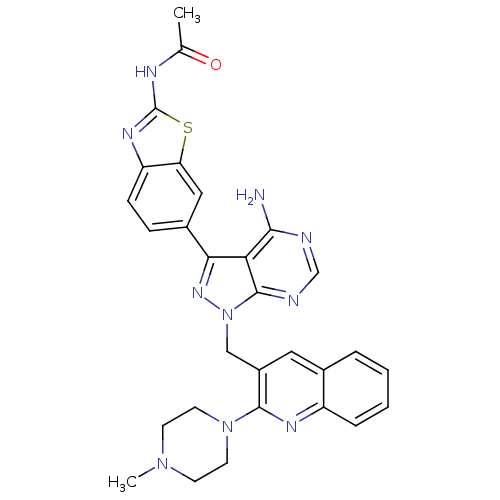
BDBM50323731 CHEMBL1213117::N-(6-(4-amino-1-((2-(4-methylpiperazin-1-yl)quinolin-3-yl)methyl)-1H-pyrazolo[3,4-d]pyrimidin-3-yl)benzo[d]thiazol-2-yl)acetamide::N-[6-(4-amino-1-{[2-(4-methylpiperazin-1-yl)quinolin-3-yl]methyl}-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzothiazol-2-yl]acetamide::US9790228, Compound 198
SMILES CN1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2ccc3nc(NC(C)=O)sc3c2)c2c(N)ncnc12
InChI Key InChIKey=PROYLAOFEKHQGS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50323731
Found 13 hits for monomerid = 50323731
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 788nMAssay Description:Inhibition of p110alphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of p110betaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 185nMAssay Description:Inhibition of p110gammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of p110deltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: <100nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta(Homo sapiens (Human))
Intellikine
US Patent
Intellikine
US Patent
Affinity DataIC50: <100nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Intellikine
US Patent
Intellikine
US Patent
Affinity DataIC50: 550nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta(Homo sapiens (Human))
Intellikine
US Patent
Intellikine
US Patent
Affinity DataIC50: <100nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: <100nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Intellikine
US Patent
Intellikine
US Patent
Affinity DataIC50: 550nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Medical Research Council-Laboratory Of Molecular Biology
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)