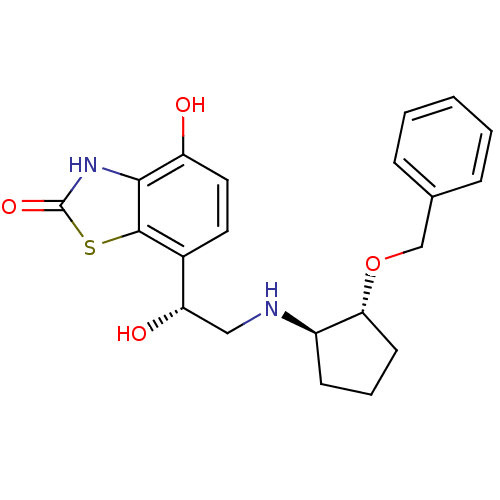
BDBM50324854 7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1-hydroxyethyl)-4-hydroxybenzo[d]thiazol-2(3H)-one::CHEMBL1221680
SMILES O[C@@H](CN[C@@H]1CCC[C@H]1OCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12
InChI Key InChIKey=QTWVYUJFBIVOHT-BPQIPLTHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50324854
Found 3 hits for monomerid = 50324854
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Displacement of [3H]CGP12177 from human beta2 adrenoceptorMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]CGP12177 from human beta-1 adrenoceptorMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 0.0316nMAssay Description:Agonist activity at beta 2 in human A431 cells adrenoceptor assessed as cAMP accumulationMore data for this Ligand-Target Pair