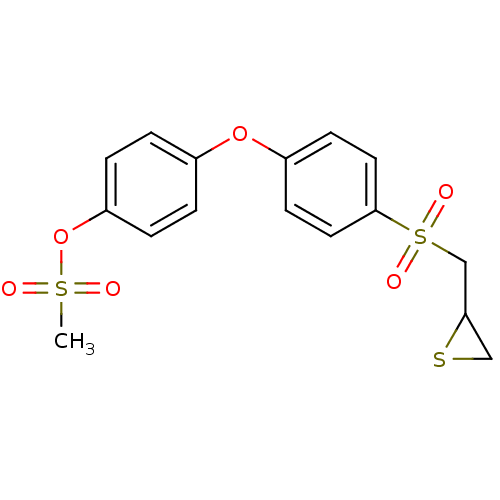
BDBM50335495 4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl methanesulfonate::CHEMBL1651842::US10357546, JNMS-38
SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1
InChI Key InChIKey=IEHSRERHOYOHOA-UHFFFAOYSA-N
Data 16 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 50335495
Found 16 hits for monomerid = 50335495
Affinity DataKi: 5nMAssay Description:Competitive inhibition of human MMP-9 assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of human recombinant MMP9 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Human recombinant active MMP-2 and MMP-7, and the catalytic domains of MMP-3 and MMP-14/MT1-MMP were purchased from EMD Chemicals, Inc. (San Diego, C...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Competitive inhibition of human MMP-2 assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 145nMAssay Description:Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 575nMAssay Description:Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of human recombinant MMP3 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Human recombinant active MMP-2 and MMP-7, and the catalytic domains of MMP-3 and MMP-14/MT1-MMP were purchased from EMD Chemicals, Inc. (San Diego, C...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of human recombinant MMP7 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.82E+4nMAssay Description:Competitive inhibition of human MMP7 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibition of human recombinant MMP1 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Competitive inhibition of human MMP1 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair