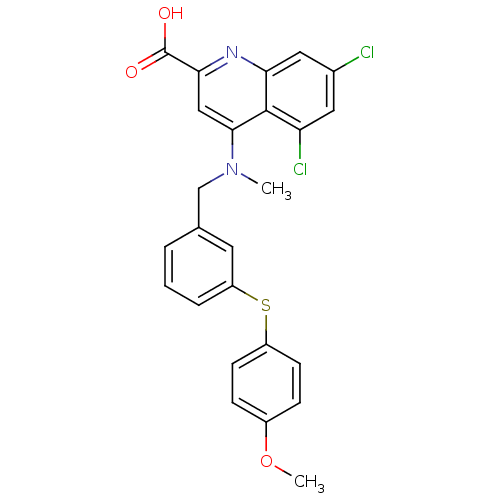
BDBM50343530 5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(methyl)amino)quinoline-2-carboxylic acid::CHEMBL1774309
SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1
InChI Key InChIKey=FUUIYLWURGZBJB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50343530
Found 9 hits for monomerid = 50343530
TargetTyrosine-protein phosphatase non-receptor type 22(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataKi: 5.53E+3nMAssay Description:Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: 6.06E+4nMAssay Description:Inhibition of STEP (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 9(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: 6.61E+4nMAssay Description:Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: 3.88E+4nMAssay Description:Inhibition of PTP1B (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: 5.07E+4nMAssay Description:Inhibition of VHR (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
TargetProtein phosphatase Slingshot homolog 2(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SSH2 (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.48E+4nMAssay Description:Inhibition of PPM1G (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 18(Homo sapiens (Human))
Shandong University
Curated by ChEMBL
Shandong University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of PTPN18 (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysisMore data for this Ligand-Target Pair