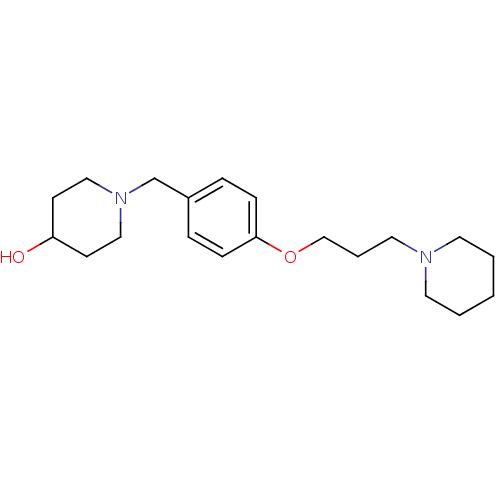
BDBM50375373 CHEMBL129306
SMILES OC1CCN(Cc2ccc(OCCCN3CCCCC3)cc2)CC1
InChI Key InChIKey=OZHWKCSDZSJHKE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50375373
Found 4 hits for monomerid = 50375373
Affinity DataKi: 0.589nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.741nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:Inhibition of histone H3 receptorMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of AChEMore data for this Ligand-Target Pair