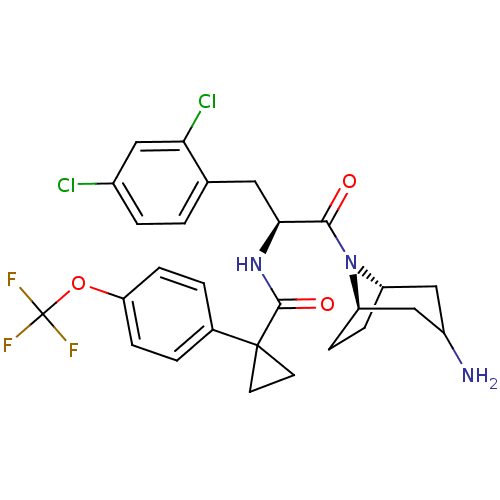
BDBM50394805 CHEMBL2163827
SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1
InChI Key InChIKey=SXVPPLOMCQSJMY-CRHVVPOVSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50394805
Found 3 hits for monomerid = 50394805
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human CYP3A4 using testosterone as substrate after 45 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assayMore data for this Ligand-Target Pair