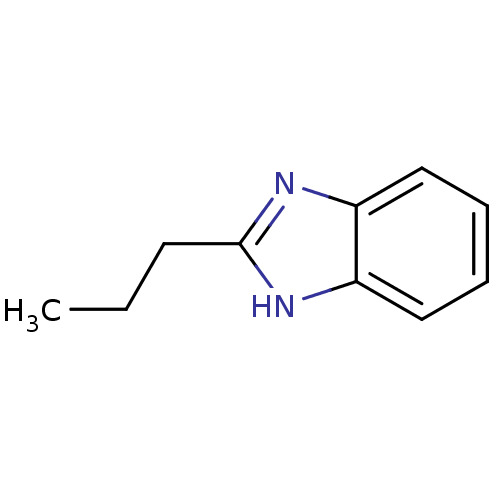
BDBM50404849 CHEMBL348508
SMILES CCCc1nc2ccccc2[nH]1
InChI Key InChIKey=FBLJZPQLNMVEMR-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50404849
Found 4 hits for monomerid = 50404849
Affinity DataIC50: 4.80E+5nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.02E+5nMAssay Description:Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats.More data for this Ligand-Target Pair
Affinity DataIC50: 4.79E+5nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated ratsMore data for this Ligand-Target Pair