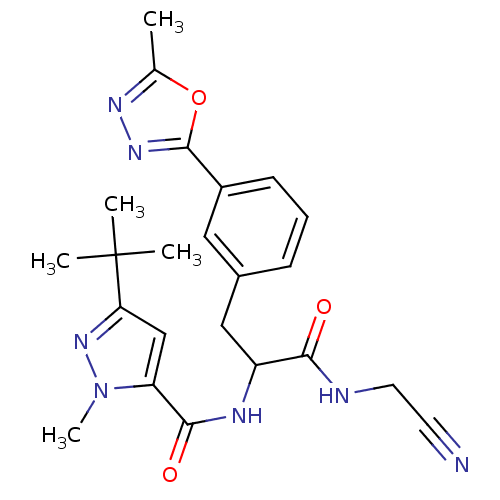
BDBM50414639 CHEMBL550872
SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1
InChI Key InChIKey=BPIZQAAUSWBFNS-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50414639
Found 5 hits for monomerid = 50414639
Affinity DataIC50: 5.01nMAssay Description:Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human liver cathepsin B assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 63.1nMAssay Description:Inhibition of cathepsin K assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair