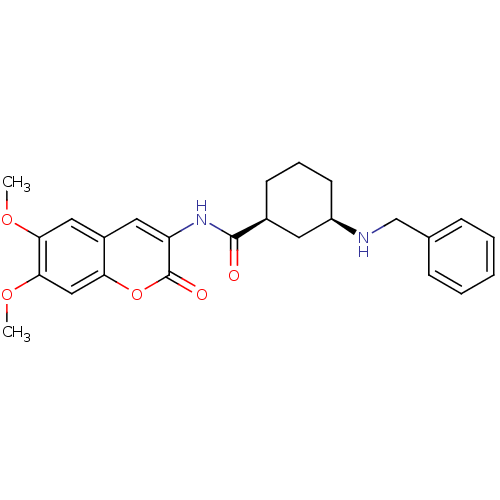
BDBM50424045 CHEMBL2314726
SMILES COc1cc2cc(NC(=O)[C@H]3CCC[C@H](C3)NCc3ccccc3)c(=O)oc2cc1OC
InChI Key InChIKey=UGTGSTDFYWUXNH-PKOBYXMFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50424045
Found 10 hits for monomerid = 50424045
Affinity DataKi: 8.60nMAssay Description:Mixed-type reversible inhibition of bovine acetylcholinesterase using S-acetylthiocholine as substrate incubated for 20 mins prior to substrate addit...More data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 93.4nMAssay Description:Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.33E+3nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.33E+3nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ...More data for this Ligand-Target Pair