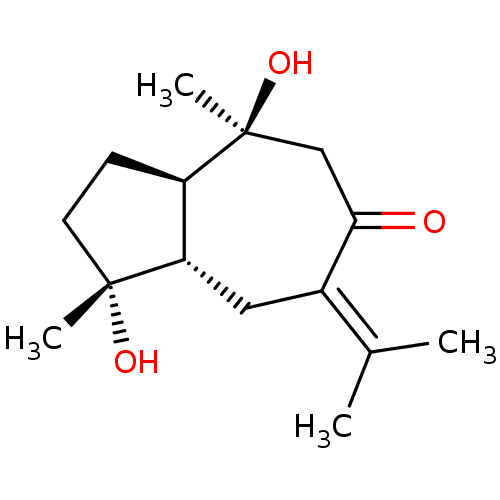
BDBM50429859 4,10-Epizedoarondiol::CHEMBL2332880
SMILES [#6]\[#6](-[#6])=[#6]-1/[#6]-[#6@H]2-[#6@@H](-[#6]-[#6][C@@]2([#6])[#8])[C@@]([#6])([#8])[#6]-[#6]-1=O
InChI Key InChIKey=TXIKNNOOLCGADE-OSRDXIQISA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50429859
Found 2 hits for monomerid = 50429859
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
University Of Toyama
Curated by ChEMBL
University Of Toyama
Curated by ChEMBL
Affinity DataIC50: 3.51E+4nMAssay Description:Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenol production from pNPP substrate after 30 mins by HTS7000 bioassay reader an...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
University Of Toyama
Curated by ChEMBL
University Of Toyama
Curated by ChEMBL
Affinity DataIC50: 1.57E+4nMAssay Description:Inhibition of TCPTP (unknown origin) assessed as decrease in p-nitrophenol production from pNPP substrate after 30 mins by HTS7000 bioassay reader an...More data for this Ligand-Target Pair