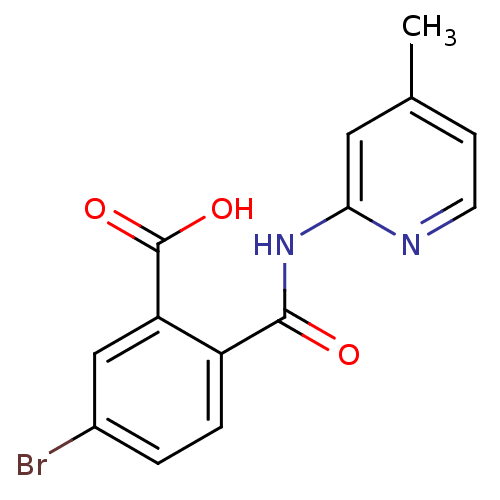
BDBM50445046 CHEMBL3098761::US10195186, Example 24::US9682967, 24
SMILES Cc1ccnc(NC(=O)c2ccc(Br)cc2C(O)=O)c1
InChI Key InChIKey=LZLNXCIQFRZLCM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50445046
Found 4 hits for monomerid = 50445046
Affinity DataKi: 2.29E+3nM IC50: 2.40E+3nMpH: 7.4Assay Description:The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ...More data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill...More data for this Ligand-Target Pair