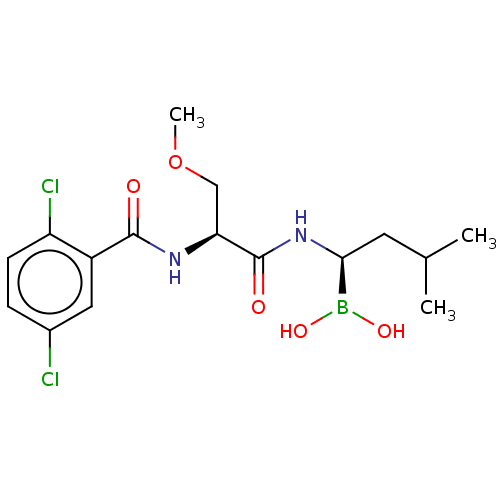
BDBM50462625 CHEMBL4243375::US11542283, Compound IV-8
SMILES COC[C@H](NC(=O)c1cc(Cl)ccc1Cl)C(=O)N[C@@H](CC(C)C)B(O)O
InChI Key InChIKey=QZRBBSWQICQORE-KBPBESRZSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50462625
Found 2 hits for monomerid = 50462625
TargetProteasome subunit alpha type-6(Homo sapiens (Human))
Jiangsu Chia Tai Fenghai Pharmaceutical
US Patent
Jiangsu Chia Tai Fenghai Pharmaceutical
US Patent
Affinity DataIC50: 7.52nMAssay Description:The proteasome used in the present invention is human erythrocyte 20S proteasome, and the enzyme, fluorescent substrate and test buffer are all purch...More data for this Ligand-Target Pair
TargetProteasome subunit beta type-5(Homo sapiens (Human))
Nanjing Forestry University
Curated by ChEMBL
Nanjing Forestry University
Curated by ChEMBL
Affinity DataIC50: 8.20nMAssay Description:Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra...More data for this Ligand-Target Pair