BDBM50468108 CHEMBL4280125::US11247985, Table 3.59
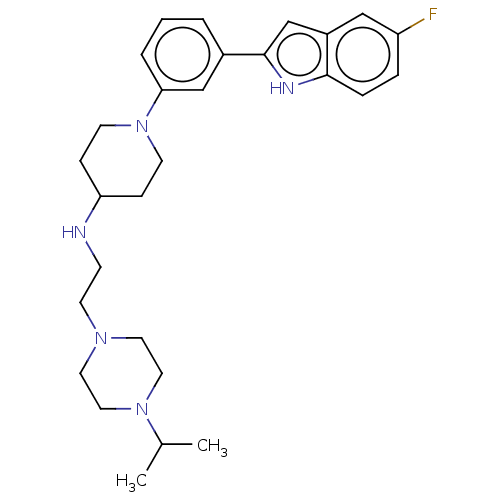
SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CC1
InChI Key InChIKey=LZHXZCVDLATFAR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50468108
Found 3 hits for monomerid = 50468108
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of recombinant full length human p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2 (DE3) using 100 uM ATP as substrate after...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
University Of Pittsburgh Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human p97More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)