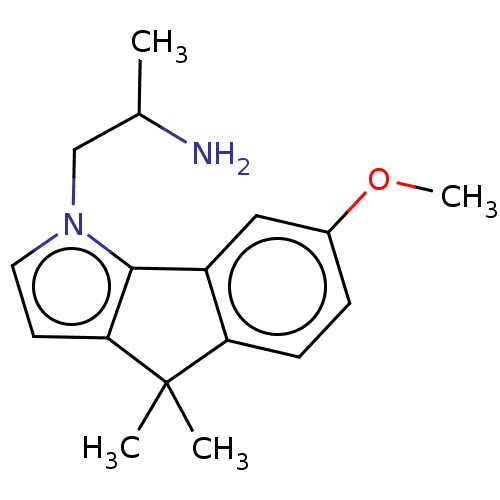
BDBM50471324 CHEMBL318639
SMILES COc1ccc2c(c1)-c1c(ccn1CC(C)N)C2(C)C
InChI Key InChIKey=BVTYPXOYRUAGFD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50471324
Found 3 hits for monomerid = 50471324
Affinity DataKi: 0.398nMAssay Description:Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOBMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity against human 5-hydroxytryptamine 2A receptor using displacement of [3H]5-HTMore data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:Efficacy (pEC50) was evaluated for 5-HT2C receptor-mediated stimulation of IP3 formation in vitro in choroid plexus of the ratMore data for this Ligand-Target Pair