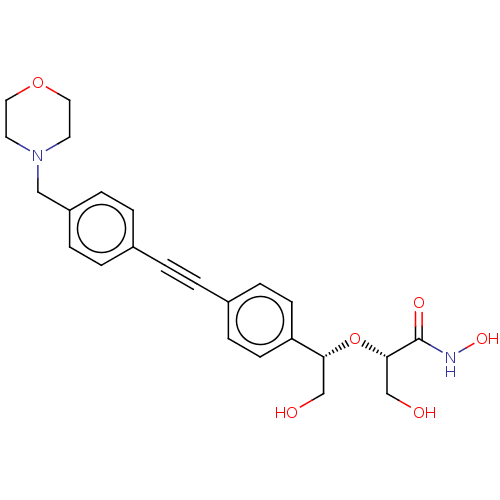
BDBM50495323 CHEMBL3103548
SMILES OC[C@H](O[C@H](CO)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO
InChI Key InChIKey=PLLPDUZTERUWMZ-PKTZIBPZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50495323
Found 2 hits for monomerid = 50495323
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Institut F£R Pharmazeutische Und Medizinische Chemie Der Westf£Lischen Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Institut F£R Pharmazeutische Und Medizinische Chemie Der Westf£Lischen Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataKi: 358nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Institut F£R Pharmazeutische Und Medizinische Chemie Der Westf£Lischen Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Institut F£R Pharmazeutische Und Medizinische Chemie Der Westf£Lischen Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add...More data for this Ligand-Target Pair