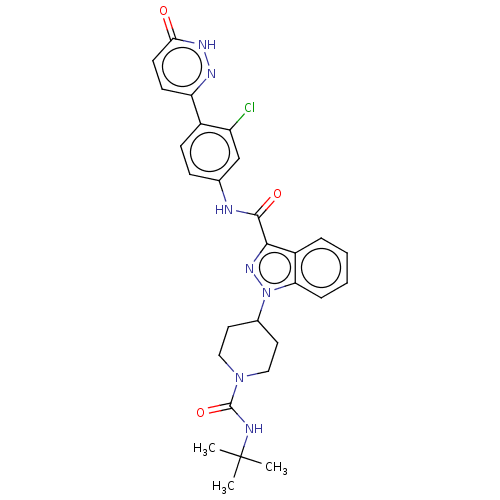
BDBM50503103 CHEMBL4555519
SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(c(Cl)c2)-c2ccc(=O)[nH]n2)c2ccccc12
InChI Key InChIKey=FBKGMJHEOILCQJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50503103
Found 3 hits for monomerid = 50503103
Affinity DataIC50: 79nMAssay Description:Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.70E+3nMAssay Description:Effect on ZAP70 phosphorylation in human Jurkat cells pre-incubated for 30 mins followed by anti-CD3/CD28 stimulation measured after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of His-tagged/GST-tagged LCK (unknown origin) incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair