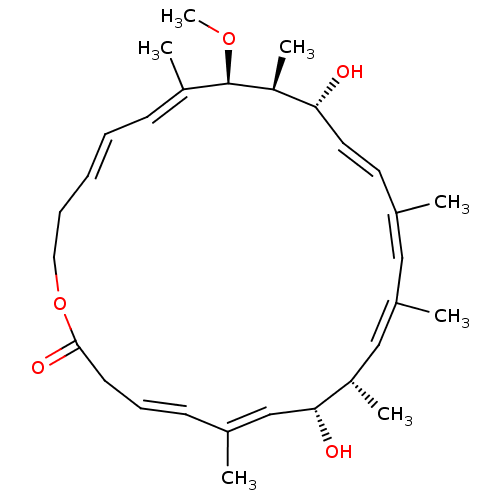
BDBM50520503 CHEMBL4471610
SMILES CO[C@H]1[C@@H](C)[C@H](O)\C=C\C(\C)=C/C(/C)=C\[C@H](C)[C@H](O)\C=C(/C)\C=C\CC(=O)OCC\C=C\C=C1/C
InChI Key InChIKey=YEYUXRUNTCBNSI-GNVMUULTSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50520503
Found 3 hits for monomerid = 50520503
Affinity DataKi: 609nMAssay Description:Displacement of [3H]PSB-11 from human A3 adenosine receptor expressed in CHO cell membranes incubated for 60 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+3nMAssay Description:Inhibition of human P2X3 assessed as reduction in agonist-induced intracellular Ca2+ concentration pre-incubated for 30 mins before agonist addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.85E+3nMAssay Description:Inhibition of human leukocyte elastase assessed as reduction in pNA release using chromogenic MeO-Suc-Ala-Ala-Pro-Val-pNA substrate measured over 10 ...More data for this Ligand-Target Pair