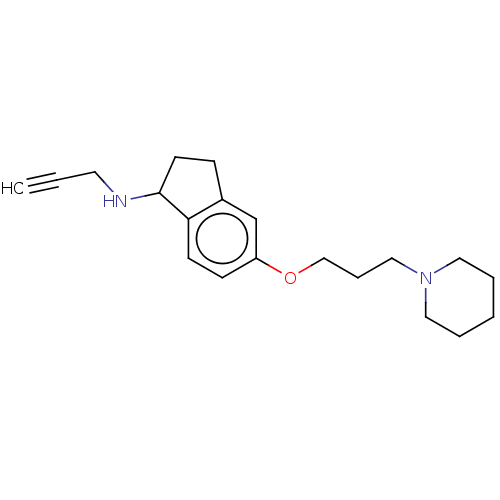
BDBM50525644 CHEMBL4545912
SMILES C#CCNC1CCc2cc(OCCCN3CCCCC3)ccc12
InChI Key InChIKey=RXNWZWWEYCPZCV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50525644
Found 12 hits for monomerid = 50525644
TargetHistamine H3 receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 minMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: >10nMAssay Description:Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 minMore data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] spiperone from human D3 dopamine receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] pyrilamine from human H1 histamine receptorMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] spiperone from human D2 dopamine receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H] histamine from human H4 histamine receptorMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 256nMAssay Description:Inhibition of human recombinant MAO-B using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant MAO-A using kynuramine as substrate preincubated for 30 mins followed by substrate addition by discontinuous fluorime...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 1.05E+3nMAssay Description:Inhibition of human recombinant MAO-B using kynuramine as substrate preincubated for 30 mins followed by substrate addition by discontinuous fluorime...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant MAO-A using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant MAO-A using kynuramine as substrate by discontinuous fluorimetric analysisMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Heinrich Heine University D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 991nMAssay Description:Inhibition of human recombinant MAO-B using kynuramine as substrate by discontinuous fluorimetric analysisMore data for this Ligand-Target Pair