BDBM50527104 CHEMBL4447583
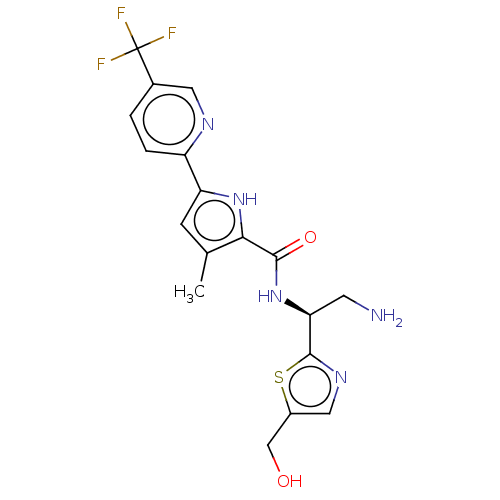
SMILES Cc1cc([nH]c1C(=O)N[C@@H](CN)c1ncc(CO)s1)-c1ccc(cn1)C(F)(F)F
InChI Key InChIKey=BILBXEUAJZPBIW-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50527104
Found 9 hits for monomerid = 50527104
TargetCytochrome P450 2C8(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 ...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.01E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 t...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 t...More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate preincubated for 5 mins followed by NADPH addition and measured for ...More data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus 1)
Rutgers University
Curated by ChEMBL
Rutgers University
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of recombinant HIV-1 reverse transcriptase incubated for 2 hrs by colorimetric assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4/3A43/3A5/3A7(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to ...More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to...More data for this Ligand-Target Pair
TargetCytochrome P450 2B6(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.54E+4nMAssay Description:Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4/3A43/3A5/3A7(Homo sapiens (Human))
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Lindsley F. Kimball Research Institute
Curated by ChEMBL
Affinity DataIC50: >6.22E+4nMAssay Description:Inhibition of CYP3A in human liver microsomes using testosterone as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)