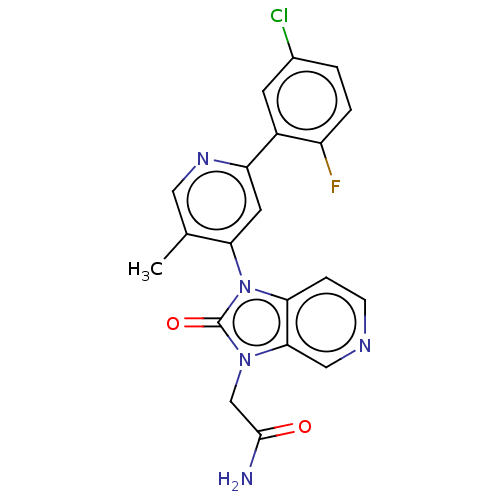
BDBM50533232 CHEMBL4564126
SMILES Cc1cnc(cc1-n1c2ccncc2n(CC(N)=O)c1=O)-c1cc(Cl)ccc1F
InChI Key InChIKey=LVEUPFUJRKZPEN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50533232
Found 10 hits for monomerid = 50533232
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 234nMAssay Description:Inhibition of ALK5 in TGF beta1-stimulated HEK293T cells by SMAD binding element-driven beta lactamase reporter gene assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 2(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of MAP3K2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 2(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of MAP3K2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of ALK5 (unknown origin) using fluorescein-labeled peptide substrate incubated for 90 mins by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of ALK5 (unknown origin) using fluorescein-labeled peptide substrate incubated for 90 mins by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 234nMAssay Description:Inhibition of ALK5 in TGF beta1-stimulated HEK293T cells by SMAD binding element-driven beta lactamase reporter gene assayMore data for this Ligand-Target Pair