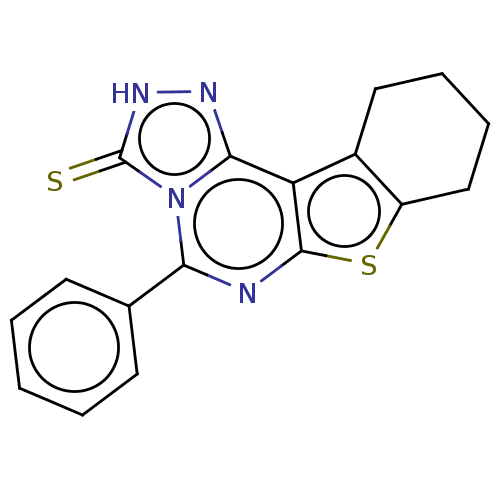
BDBM50533823 CHEMBL494210
SMILES S=c1[nH]nc2c3c4CCCCc4sc3nc(-c3ccccc3)n12
InChI Key InChIKey=PQXOHDIQJFCWFC-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50533823
Found 3 hits for monomerid = 50533823
Affinity DataKi: 5.04E+3nMAssay Description:Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair