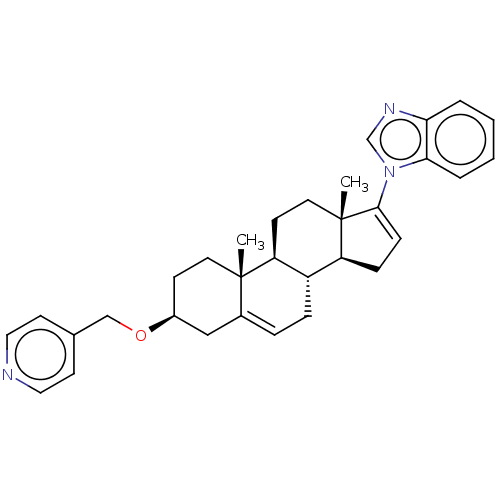
BDBM50536421 CHEMBL4541575
SMILES [H][C@@]12CC=C(n3cnc4ccccc34)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OCc1ccncc1
InChI Key InChIKey=BQCWJFBXMLBWKK-CIISATOMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50536421
Found 2 hits for monomerid = 50536421
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Inhibition of CYP17 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 1.65E+3nMAssay Description:Inhibition of DHT-induced androgen receptor transactivation in human LNCaP cells after 24 hrs by luciferase reporter gene assay relative to controlMore data for this Ligand-Target Pair