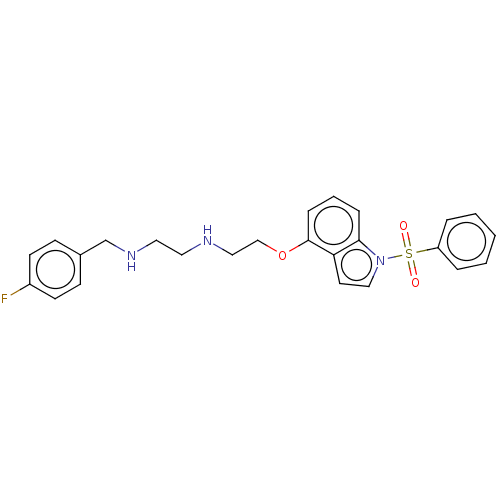
BDBM50579331 CHEMBL4852099
SMILES Fc1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1
InChI Key InChIKey=PMICITQTHHHUOJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50579331
Found 3 hits for monomerid = 50579331
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 6.21E+6nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ...More data for this Ligand-Target Pair