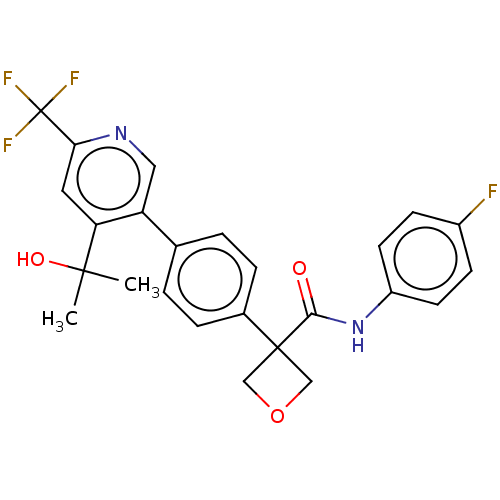
BDBM50604026 CHEMBL5192384
SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F
InChI Key InChIKey=ICJRFPZBMMQAPU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50604026
Found 5 hits for monomerid = 50604026
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H] labeled-N-(4-Fluorophenyl)-3-(4-(4-(2-hydroxypropan-2-yl)-6-(trifluoromethyl)pyridin-3-yl)phenyl)oxetane-3-carboxamide from huma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS stimulated human whole blood assessed as unbound concentration using kynurenine/tryptophan as substrate preincubat...More data for this Ligand-Target Pair
Affinity DataIC50: 8.64E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of IDO1 in IFN gamma-stimulated human HeLa cells measured after 48 hrs by fluorescence based microplate reader assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pharmaron Beijing
Curated by ChEMBL
Pharmaron Beijing
Curated by ChEMBL
Affinity DataIC50: 2.13E+7nMAssay Description:Inhibition of MK-499 binding to hERGMore data for this Ligand-Target Pair