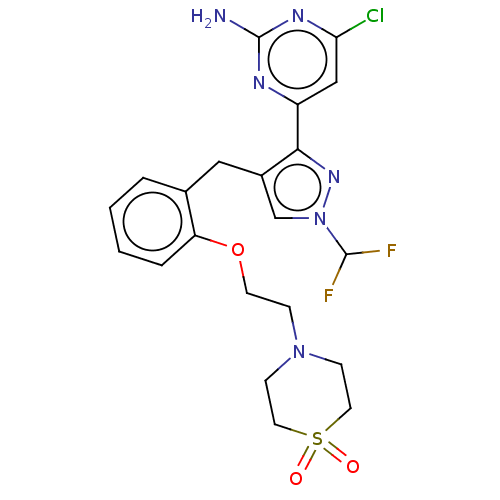
BDBM50607679 CHEMBL5218615
SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCS(=O)(=O)CC1)C(F)F
InChI Key InChIKey=NMBRYEUXWVHUEM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50607679
Found 3 hits for monomerid = 50607679
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataKd: 2.10nMAssay Description:Binding affinity to human recombinant His-tagged ADCY10 assessed as dissociation constant and measured for 600 sec by SPR analysisMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair