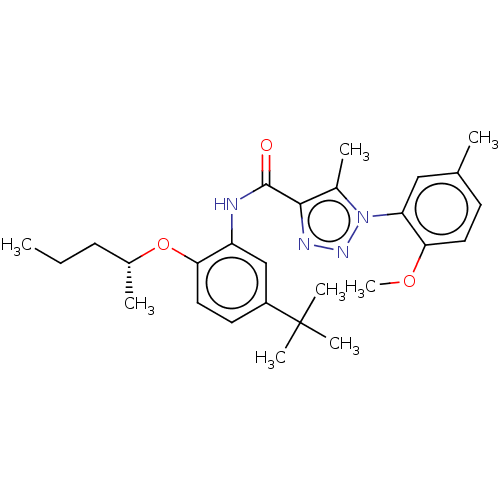
BDBM50609819 CHEMBL5274748
SMILES CCC[C@@H](C)Oc1ccc(cc1NC(=O)c1nnn(c1C)-c1cc(C)ccc1OC)C(C)(C)C
InChI Key InChIKey=UOXKTTLWMUQDFC-GOSISDBHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50609819
Found 3 hits for monomerid = 50609819
Affinity DataIC50: 11nMAssay Description:Inhibition of BODIPY FL vindoline binding to recombinant human GST-tagged PXR LBD (111 to 434 residues) expressed in baculovirus infected insect cell...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 760nMAssay Description:Antagonist activity at human PXR in human HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter assessed as inhibition in rifamp...More data for this Ligand-Target Pair
Ligand Info
Affinity DataEC50: 8.10nMAssay Description:Agonist activity at human PXR in human HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter assessed as transcriptional activat...More data for this Ligand-Target Pair
Ligand Info