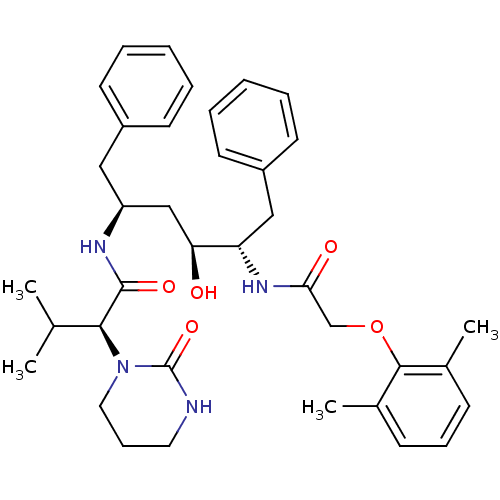
BDBM578 (2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide::ABT-378::Aluviran::CHEMBL729::LPV::Lopinavir
SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1
InChI Key InChIKey=KJHKTHWMRKYKJE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 578
Found 23 hits for monomerid = 578
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.00100nMAssay Description:Inhibitory activity against HIV proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.00400nMAssay Description:Binding affinity against ritonavir-resistant strains.More data for this Ligand-Target Pair
Affinity DataKi: 0.00500nMAssay Description:Inhibition assay using HIV protease and Sulfonamide compounds.More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [491-589,Q496K](Human immunodeficiency virus type 1)
University of Massachusetts Medical School
University of Massachusetts Medical School
Affinity DataKi: 0.00500nMAssay Description:HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 0.0190nM ΔG°: -15.2kcal/molepH: 4.7 T: 2°CAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [491-589,Q498K,D521N,V555I,N579D](Human immunodeficiency virus type 1)
University of Massachusetts Medical School
University of Massachusetts Medical School
Affinity DataKi: 0.0400nMAssay Description:HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 0.0500nM ΔG°: -14.6kcal/molepH: 4.7 T: 2°CAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition constant for human immunodeficiency virus type 1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKd: 0.100nMAssay Description:Binding affinity for human immunodeficiency virus type 1 proteaseMore data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,V583F](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 0.200nM ΔG°: -13.8kcal/molepH: 4.7 T: 2°CAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 0.480nMAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 0.5nMAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [491-589,Q498K,L501I,V555I,A562V,G564S,I575V,L581M](Human immunodeficiency virus type 1)
University of Massachusetts Medical School
University of Massachusetts Medical School
Affinity DataKi: 0.900nMAssay Description:HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [491-589,Q498K,L501I,G539V,I545V,V555I,V573A](Human immunodeficiency virus type 1)
University of Massachusetts Medical School
University of Massachusetts Medical School
Affinity DataKi: 6.10nMAssay Description:HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M](Human immunodeficiency virus type 1)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataKi: 9nMAssay Description:The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 35nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:TP_TRANSPORTER: inhibition of Rhodamine 123 efflux in Caco-2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.51E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.84E+4nMAssay Description:Inhibition of mouse ZMPSTE24 expressed n delta ste24 delta rce1 yeastMore data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 6 hits for monomerid = 578
Found 6 hits for monomerid = 578
ITC DataΔG°: -15.1kcal/mole −TΔS°: -11.3kcal/mole ΔH°: -3.79kcal/mole logk: 1.20E+11
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C
ITC DataΔG°: -11.3kcal/mole −TΔS°: -15.6kcal/mole ΔH°: 4.29kcal/mole logk: 1.80E+8
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C
ITC DataΔG°: -12.8kcal/mole −TΔS°: -15.6kcal/mole ΔH°: 2.80kcal/mole logk: 2.50E+9
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C
CellHIV-1 Protease Mutant (V82A/I84V)(Human immunodeficiency virus type 1)
The Johns Hopkins University
The Johns Hopkins University
ITC DataΔG°: -13.9kcal/mole −TΔS°: -11.8kcal/mole ΔH°: -2.10kcal/mole logk: 1.60E+10
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C
CellHIV-1 Protease Mutant (M46I/I54V)(Human immunodeficiency virus type 1)
The Johns Hopkins University
The Johns Hopkins University
ITC DataΔG°: -14.9kcal/mole −TΔS°: -14.9kcal/mole ΔH°: 0kcal/mole logk: 8.10E+10
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C
CellHIV-1 Protease Mutant (L10I/L90M)(Human immunodeficiency virus type 1)
The Johns Hopkins University
The Johns Hopkins University
ITC DataΔG°: -14.9kcal/mole −TΔS°: -13.3kcal/mole ΔH°: -1.60kcal/mole logk: 8.70E+10
pH: 5.0 T: 25.00°C
pH: 5.0 T: 25.00°C