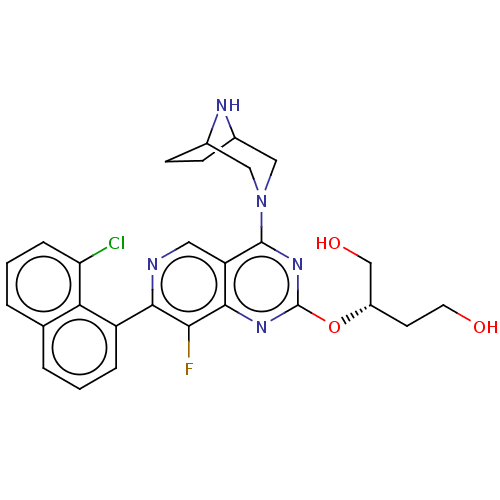
BDBM616996 US20230279025, Example 58
SMILES OCC[C@@H](CO)Oc1nc(N2CC3CCC(C2)N3)c2cnc(c(F)c2n1)-c1cccc2cccc(Cl)c12
InChI Key InChIKey=CMWHOTNLOFKJPK-ABHNRTSZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 616996
Found 2 hits for monomerid = 616996
Affinity DataIC50: 2.33E+3nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataKd: 4.73E+3nMAssay Description:Briefly, 1L of 1.05X HBS-Mg buffer (262.5 mM Bioultra Hepes, pH 7.5, 157.5 mM NaCl, 105 mM MgCl2, 0.525 mM TCEP, 0.0305% Brij-35) was prepared and fi...More data for this Ligand-Target Pair