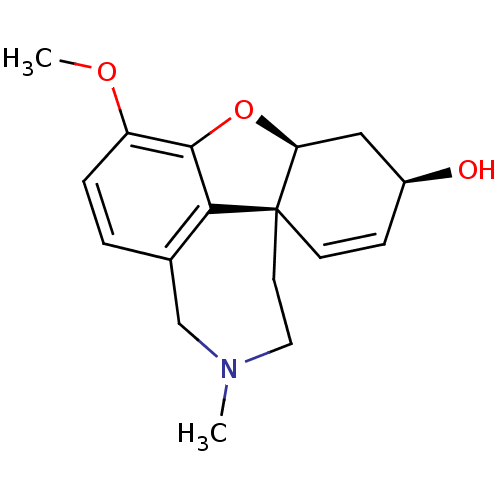
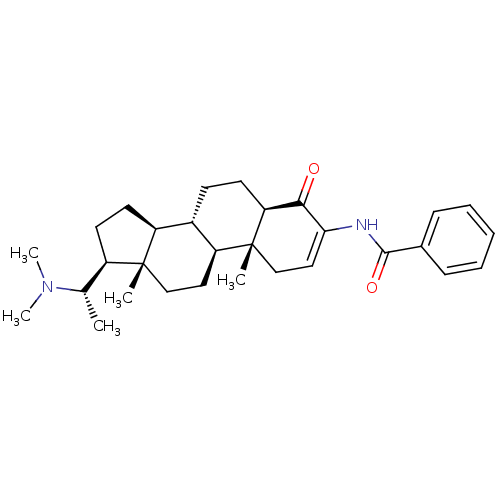
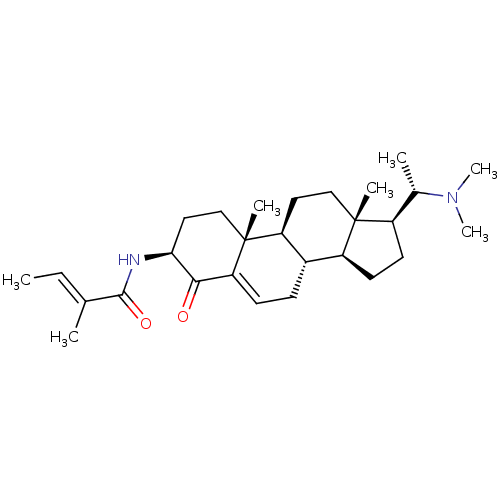
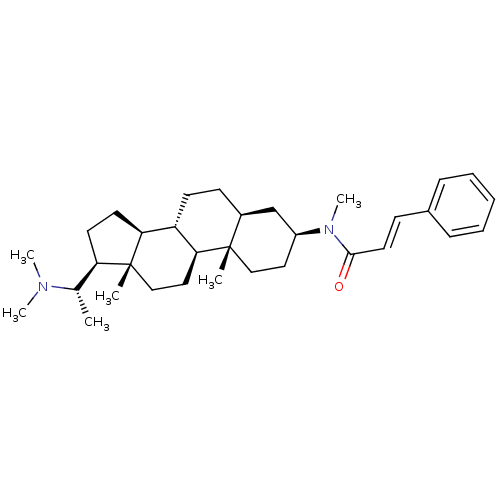
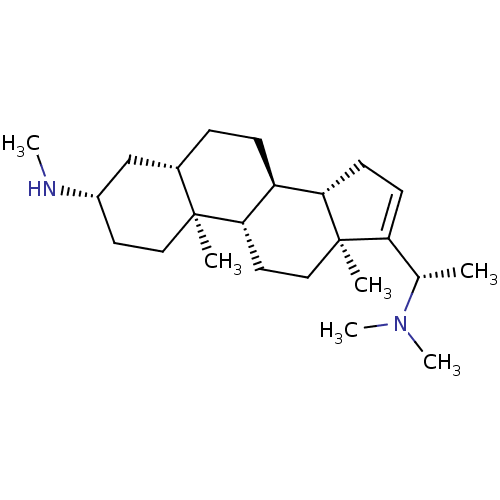
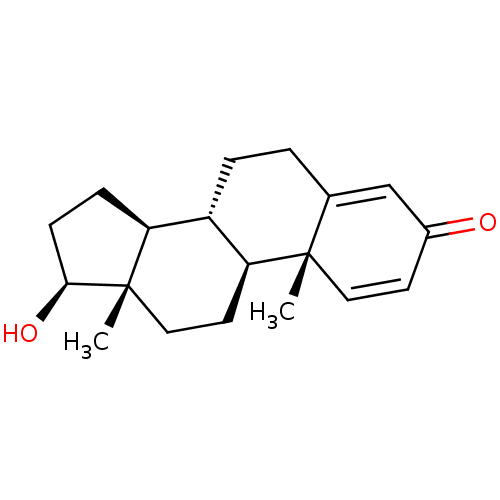
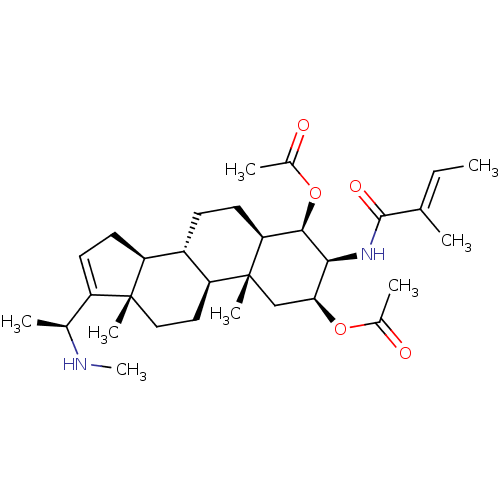
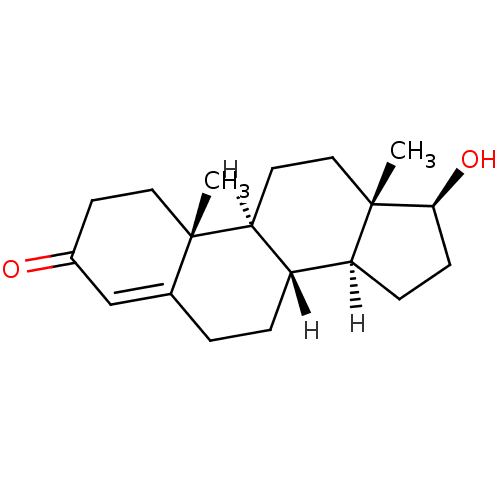
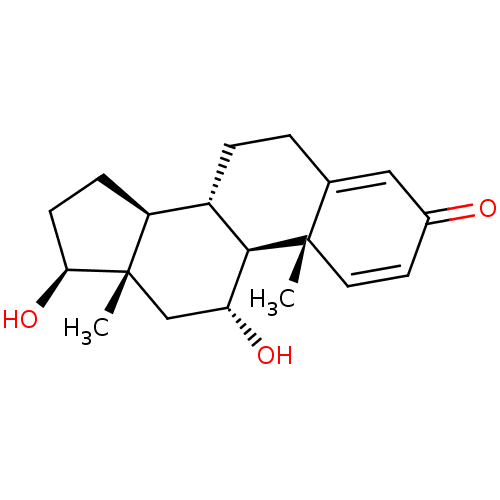
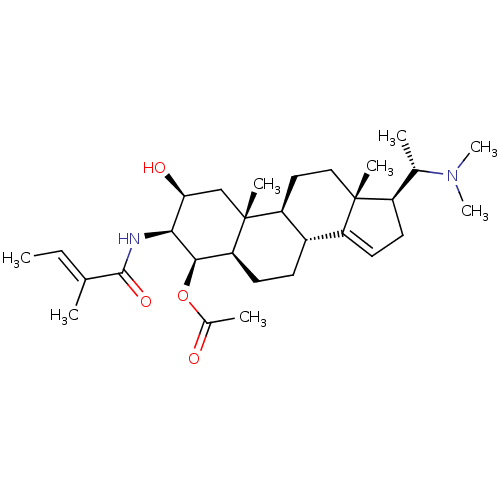
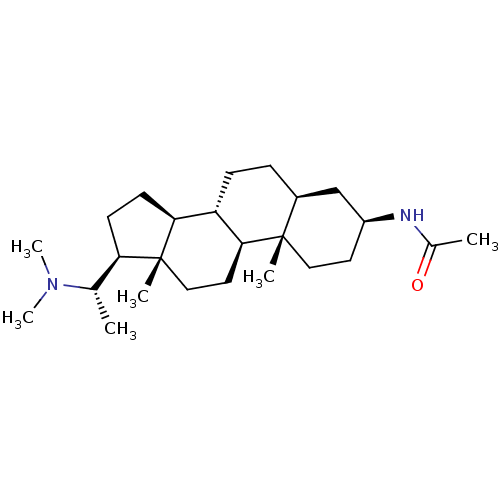
Affinity DataKi: 25nM ΔG°: -44.1kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University of Karachi
University of Karachi
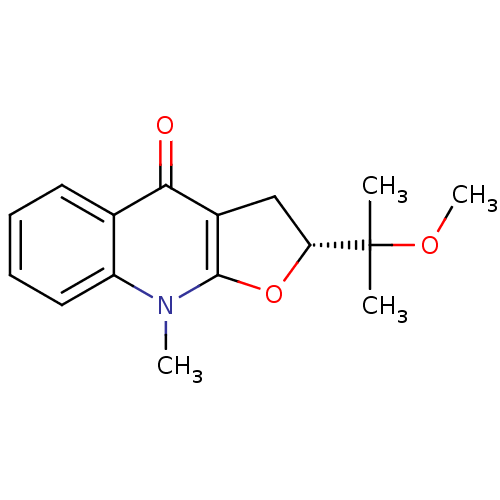
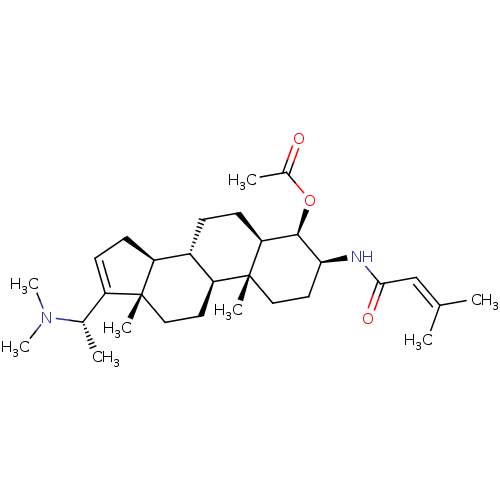
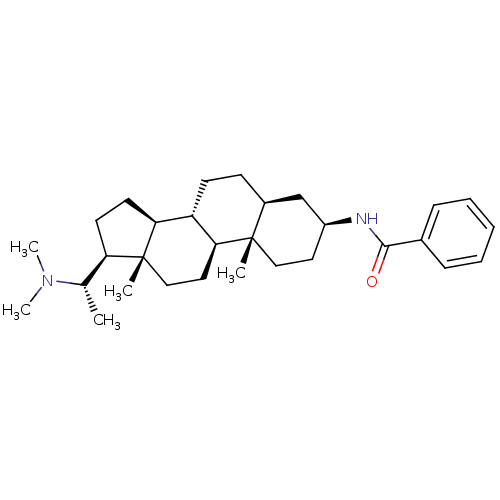
Affinity DataKi: 190nM ΔG°: -39.0kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University of Karachi
University of Karachi
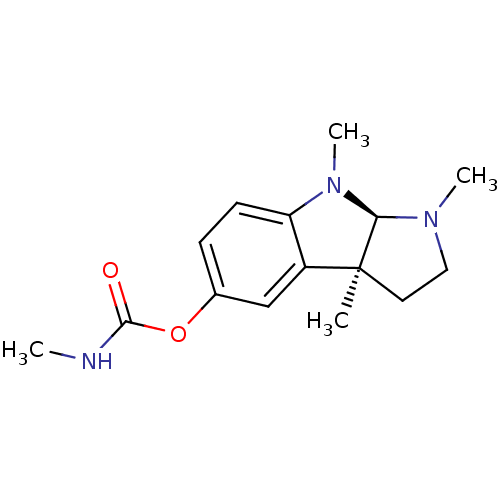
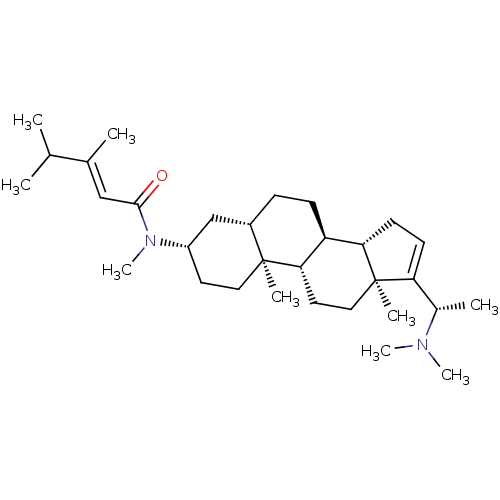
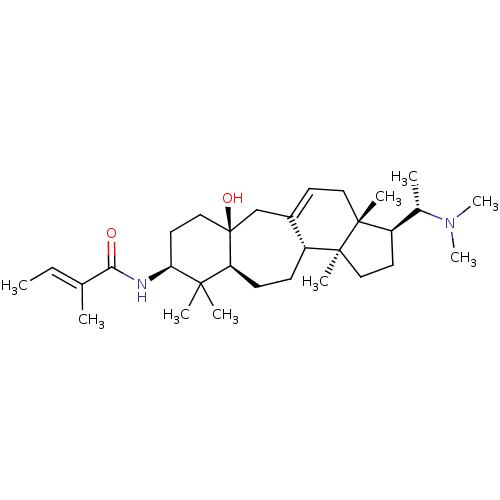
Affinity DataKi: 230nM ΔG°: -38.5kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
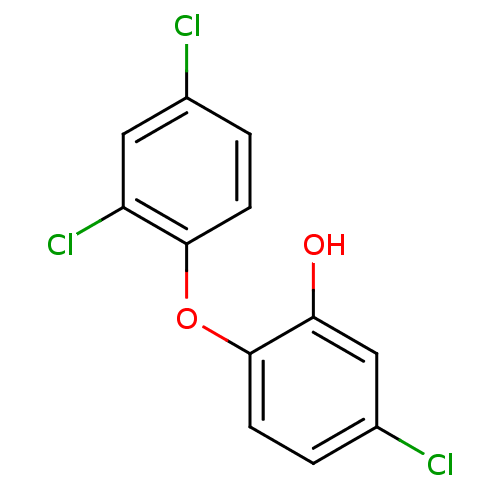
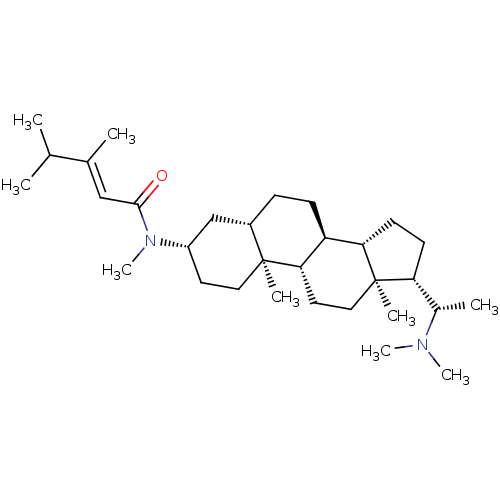
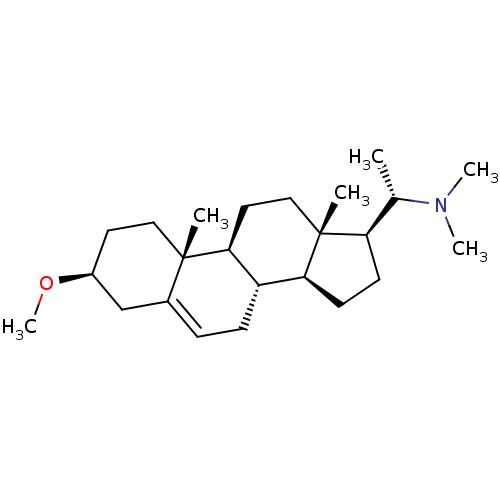
Affinity DataKi: 1.90E+4nM ΔG°: -27.4kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University of Karachi
University of Karachi
Affinity DataKi: 3.00E+4nM ΔG°: -26.2kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University of Karachi
University of Karachi
Affinity DataKi: 3.00E+4nM ΔG°: -26.2kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nM ΔG°: -26.1kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+4nM ΔG°: -24.1kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nM ΔG°: -23.5kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University of Karachi
University of Karachi
Affinity DataKi: 1.10E+5nM ΔG°: -23.0kJ/moleT: 2°CAssay Description:AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tribhuvan University
Curated by ChEMBL
Tribhuvan University
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tribhuvan University
Curated by ChEMBL
Tribhuvan University
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of electric eel AChE by modified Ellman methodMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
University Of Karachi
Curated by ChEMBL
University Of Karachi
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of Plasmodium falciparum enoyl-ACP reductase assessed as oxidation of NADH to NAD+ after 10 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 857nMAssay Description:Inhibition of horse BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 857nMAssay Description:Inhibition of BChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair
Affinity DataIC50: 5.25E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.25E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.75E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.17E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.17E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.46E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.46E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.08E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.76E+3nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of horse BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.95E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.09E+4nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of horse BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 2.88E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.02E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.12E+4nMAssay Description:Inhibition of BChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.31E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.27E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tribhuvan University
Curated by ChEMBL
Tribhuvan University
Curated by ChEMBL
Affinity DataIC50: 4.69E+4nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tribhuvan University
Curated by ChEMBL
Tribhuvan University
Curated by ChEMBL
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.35E+4nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair
Affinity DataIC50: 5.37E+4nMAssay Description:Inhibition of BChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.15E+4nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair
Affinity DataIC50: 6.17E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.92E+4nMAssay Description:Compound was tested for the in silico inhibition of acetylcholinesteraseMore data for this Ligand-Target Pair

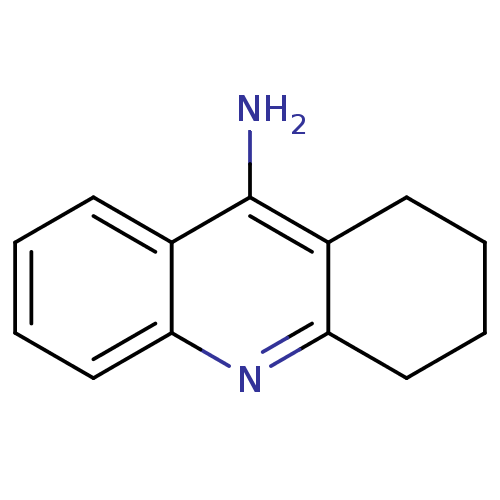
 3D Structure (crystal)
3D Structure (crystal)