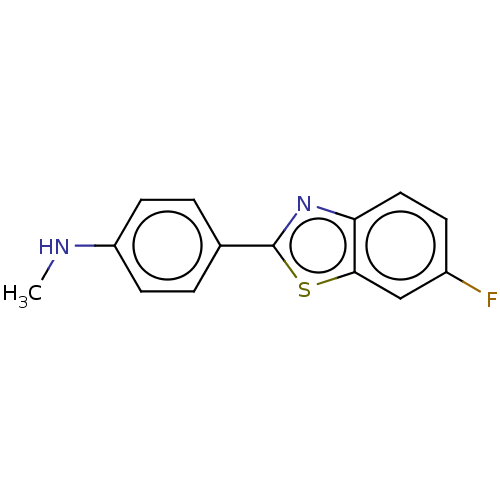
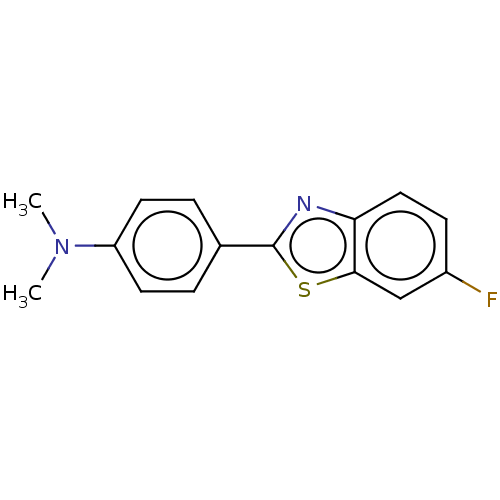
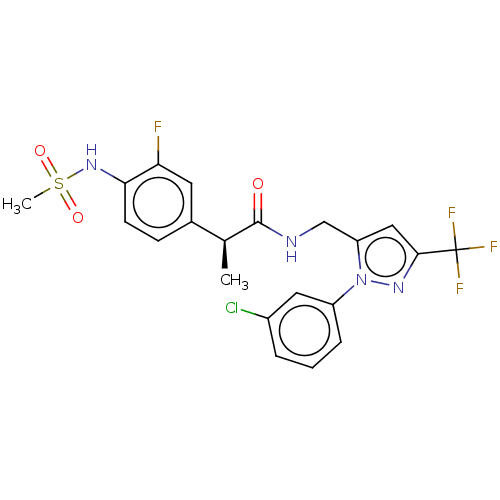
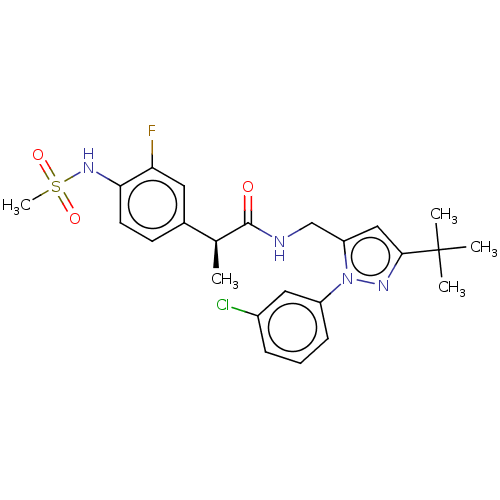
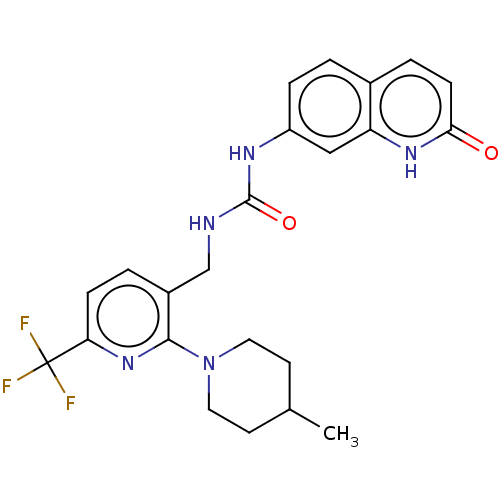
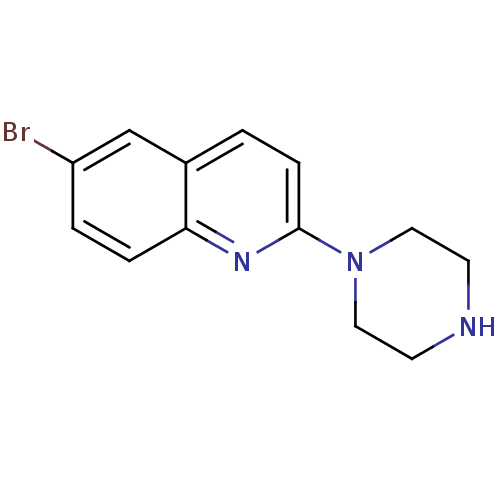
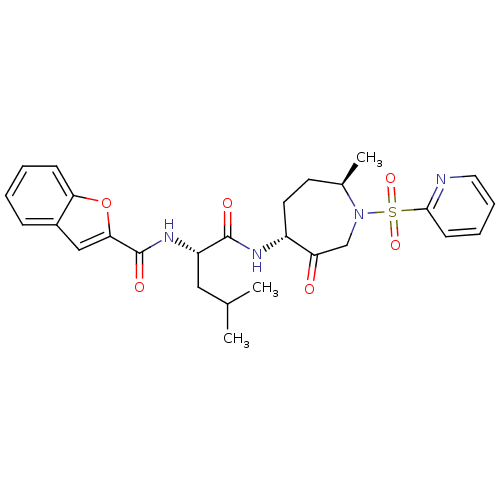
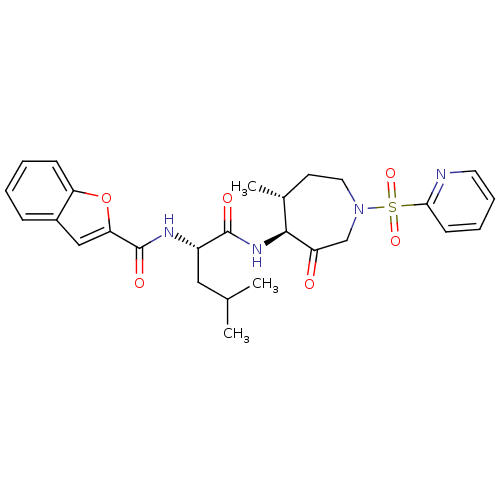
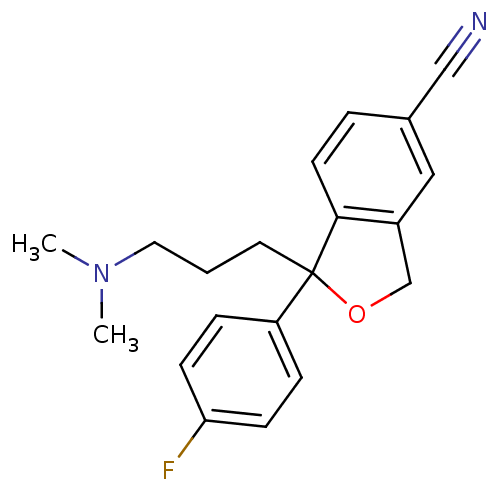
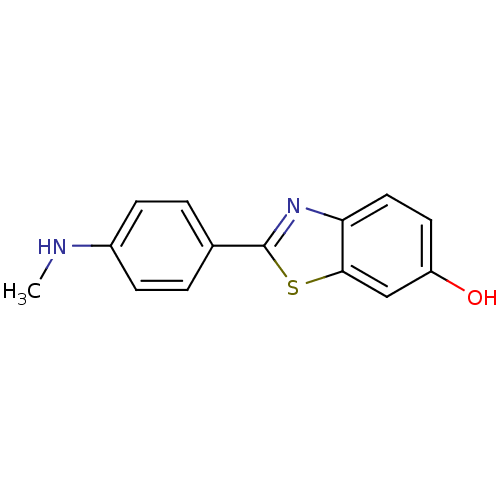
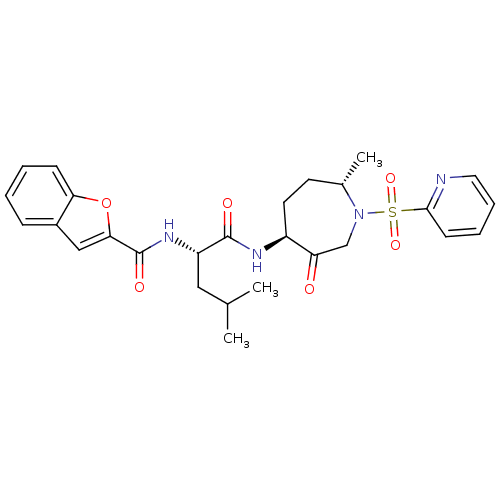
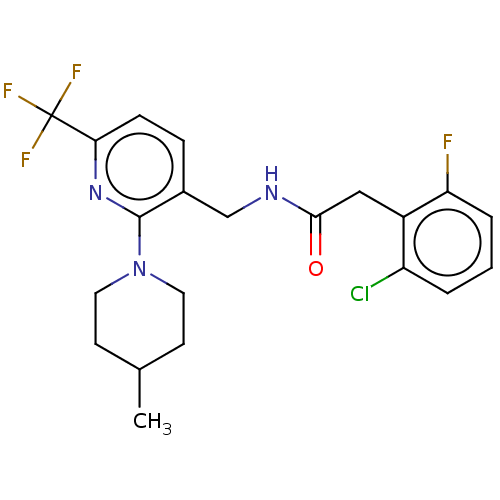
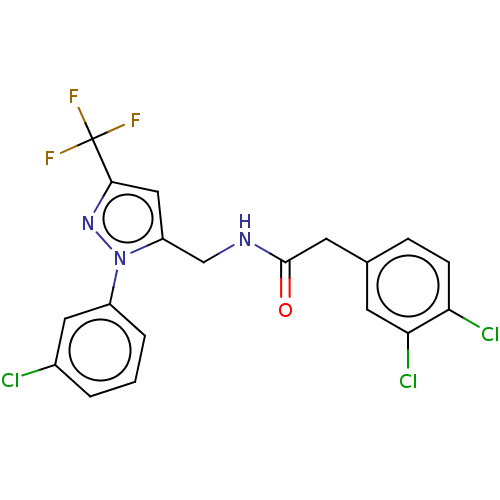
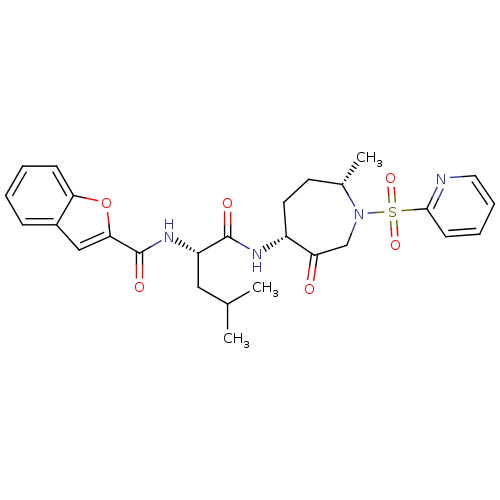
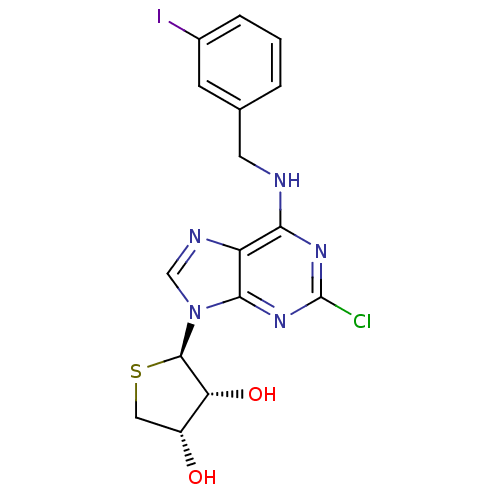
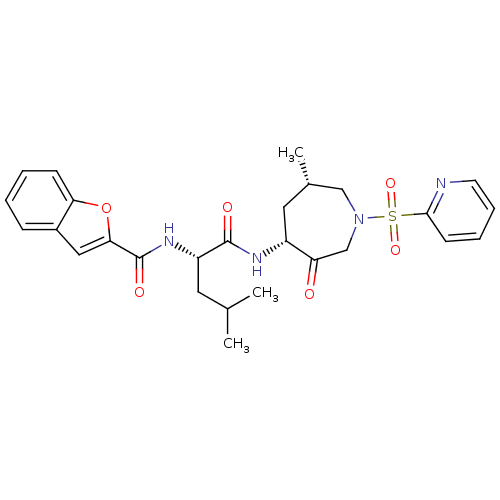
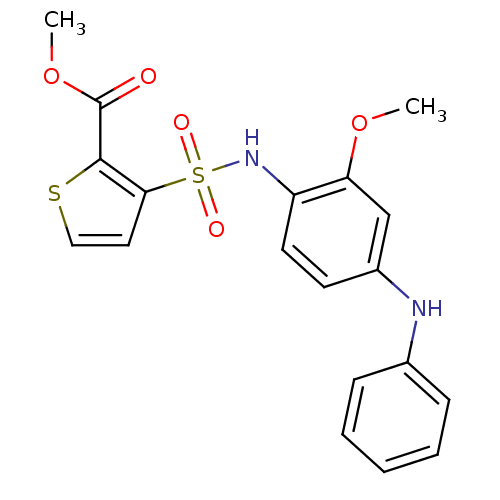
Affinity DataKi: 0.00990nM ΔG°: -62.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -58.8kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nM ΔG°: -58.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0630nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0680nM ΔG°: -57.4kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM ΔG°: -55.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.3kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Seoul National University Bundang Hospital
Curated by ChEMBL
Seoul National University Bundang Hospital
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma countingMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Seoul National University Bundang Hospital
Curated by ChEMBL
Seoul National University Bundang Hospital
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma countingMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 0.530nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nM ΔG°: -52.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.720nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 0.830nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of 30 nM capsaicin-induced calcium uptakeMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 0.910nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of 30 nM capsaicin-induced calcium uptakeMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -50.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nM ΔG°: -49.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Seoul National University Bundang Hospital
Curated by ChEMBL
Seoul National University Bundang Hospital
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma countingMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Inha University
Curated by ChEMBL
Inha University
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -48.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonist activity at human TRPV1 expressed in HEK cells assessed as inhibition of 10 nM capsaicin-induced calcium uptake by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -48.6kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of 30 nM capsaicin-induced calcium uptakeMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of 30 nM capsaicin-induced calcium uptakeMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Displacement of [125I]I-AB-MECA from recombinant human A3AR expressed in CHO cell membranes measured after 60 mins by gamma counter analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nM ΔG°: -47.3kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4.5nM ΔG°: -47.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4.5nM ΔG°: -47.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 4.60nMAssay Description:Binding affinity to PPAR-delta (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR methodMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Seoul National University Bundang Hospital
Curated by ChEMBL
Seoul National University Bundang Hospital
Curated by ChEMBL
Affinity DataKi: 5.5nMAssay Description:Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma countingMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Seoul National University Bundang Hospital
Curated by ChEMBL
Seoul National University Bundang Hospital
Curated by ChEMBL
Affinity DataKi: 5.80nMAssay Description:Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma countingMore data for this Ligand-Target Pair

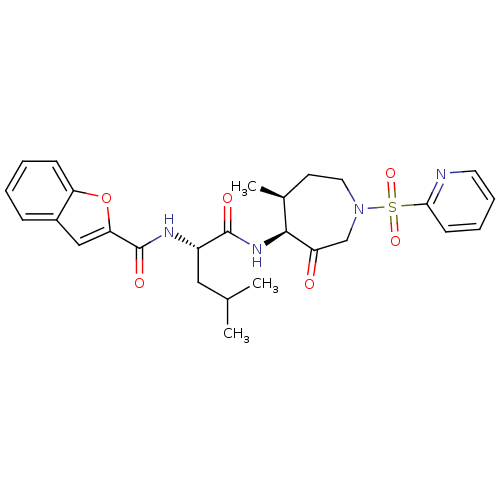
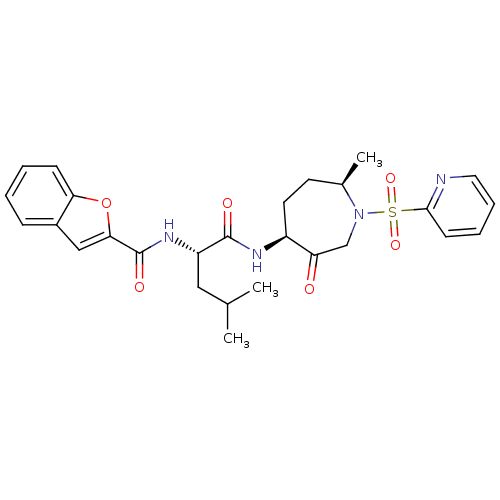
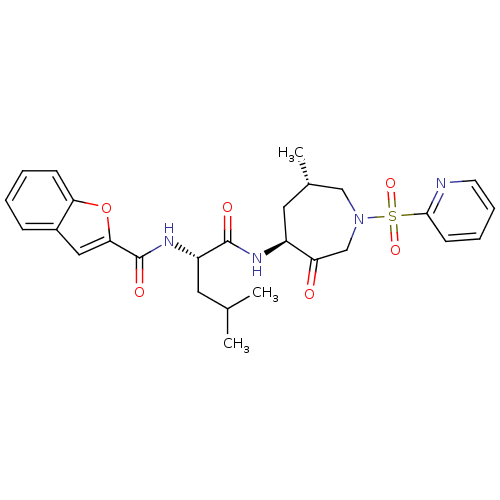

 3D Structure (crystal)
3D Structure (crystal)