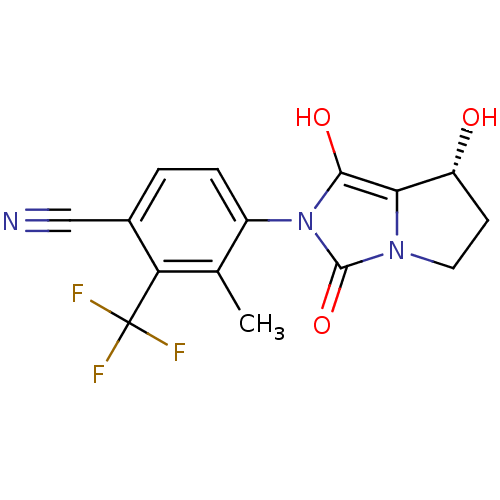
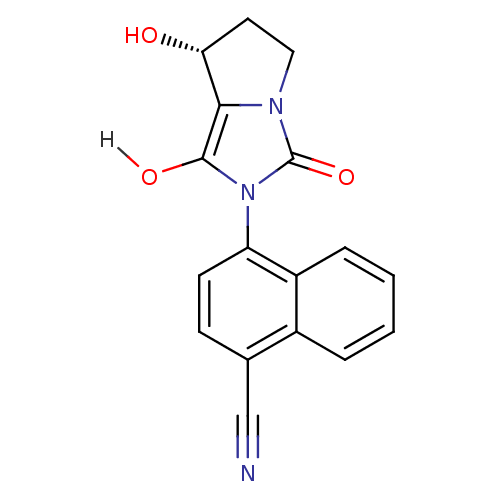
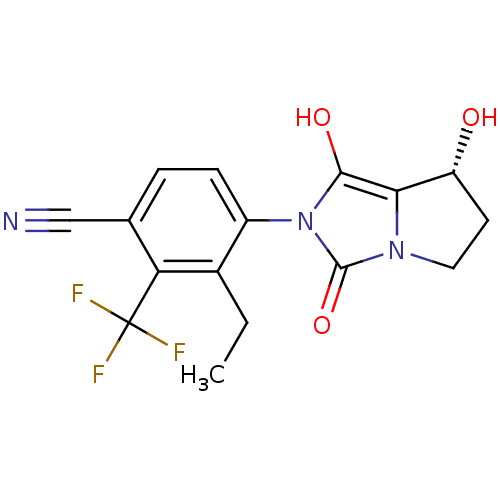
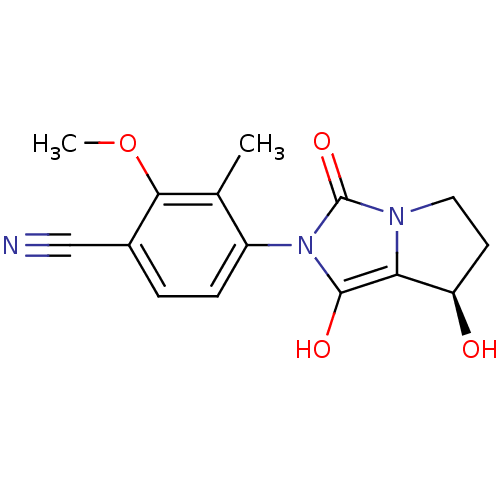
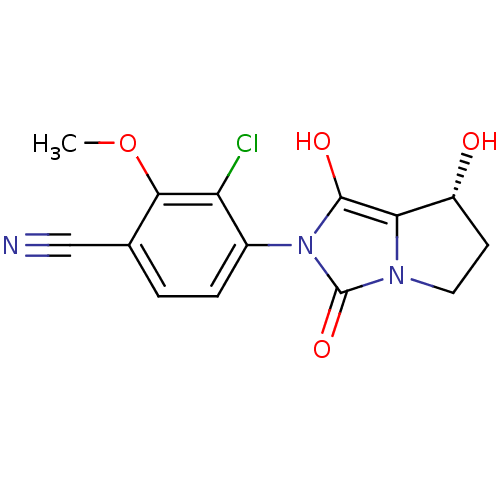
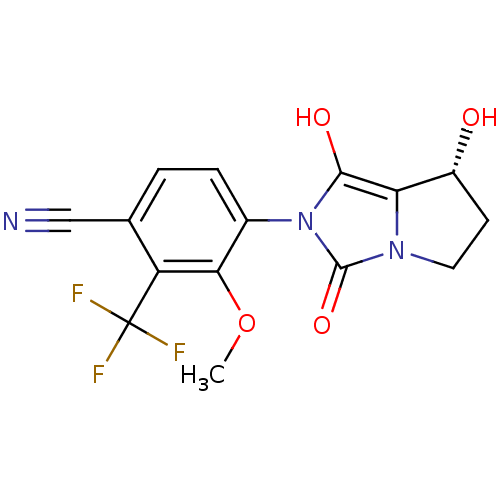
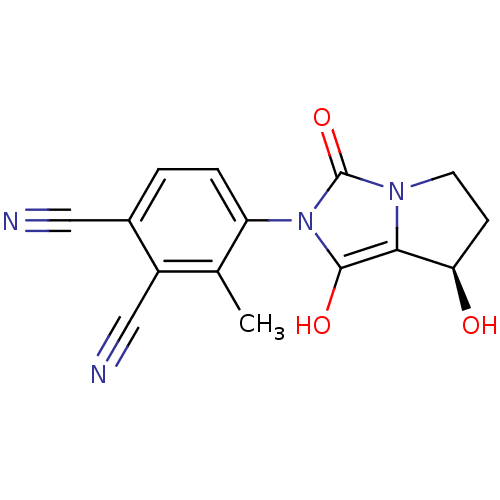
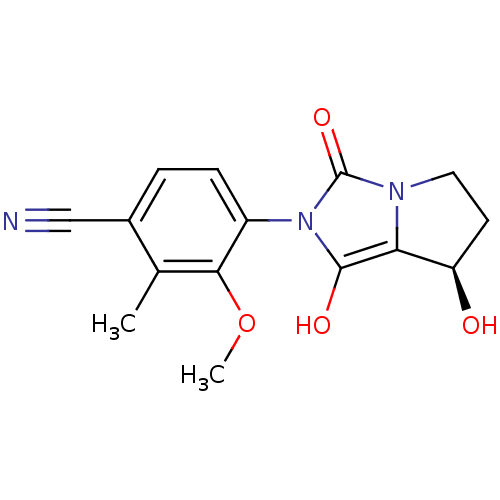
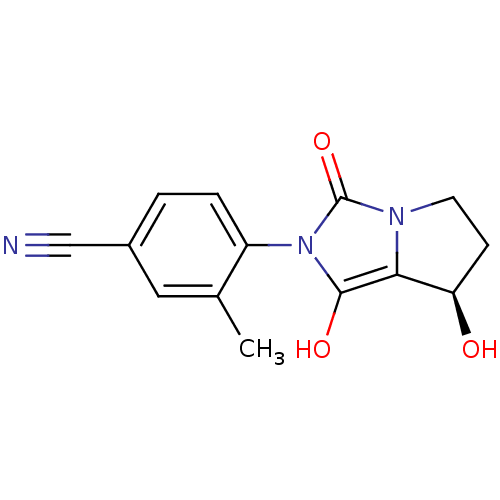
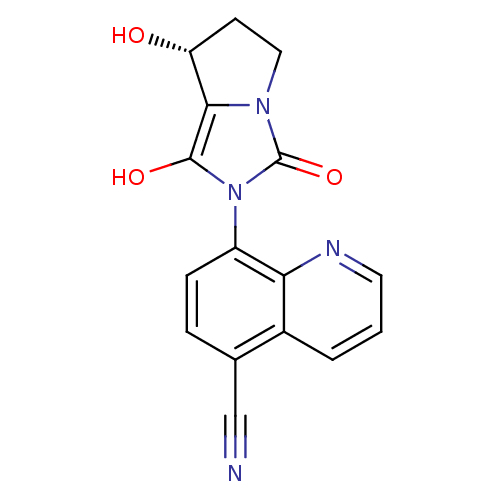
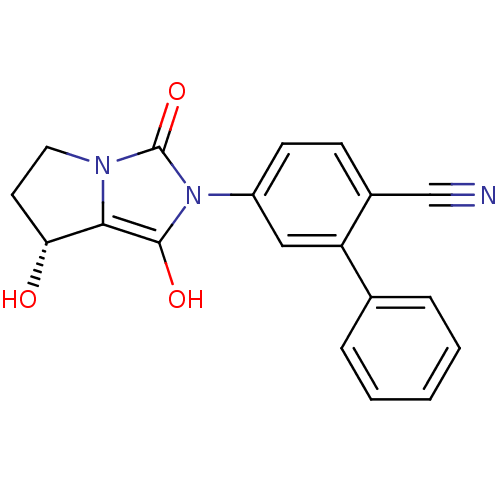
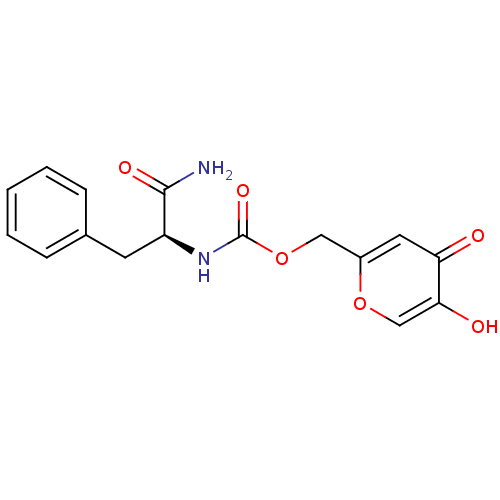
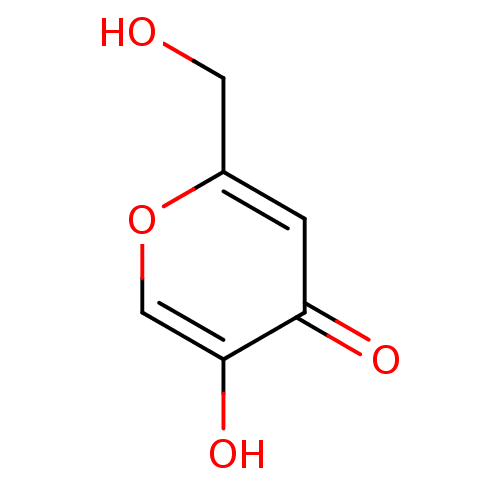
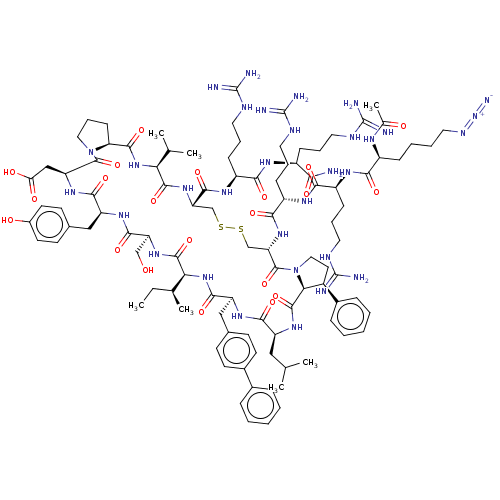
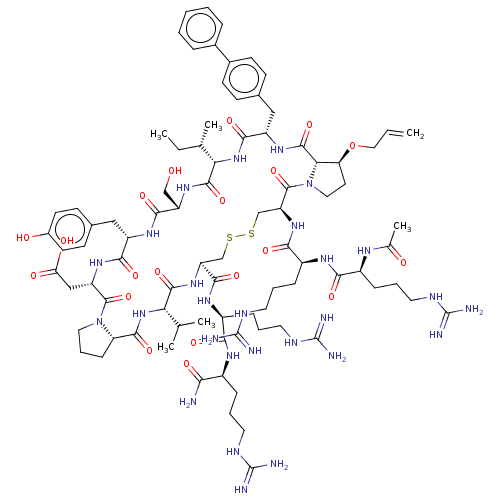
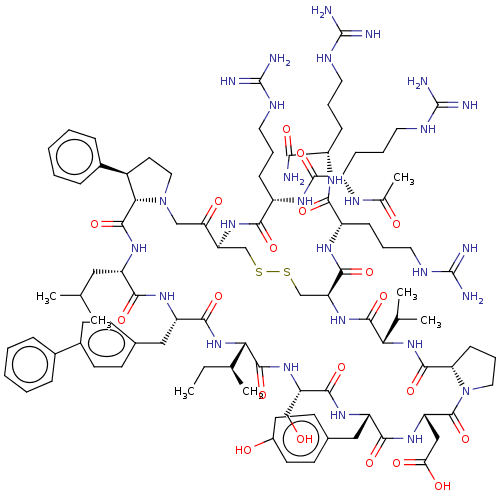
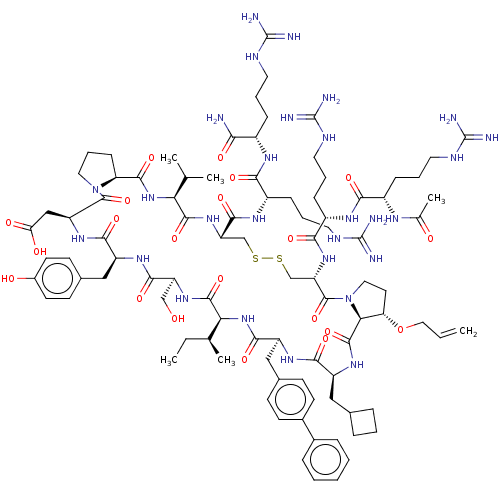
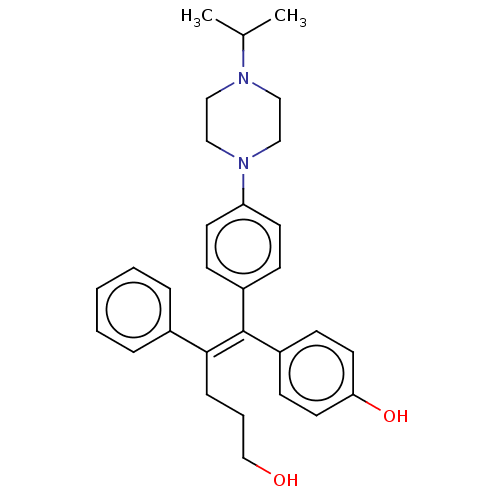
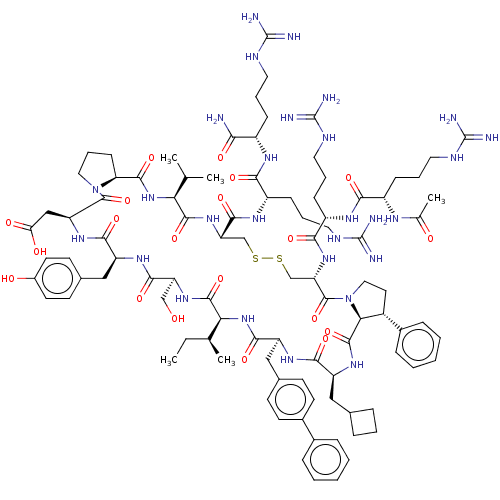
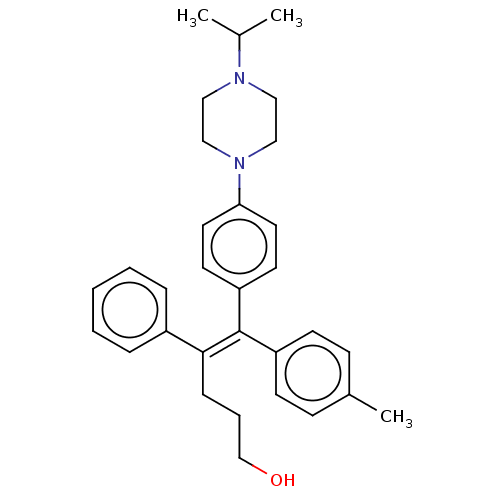
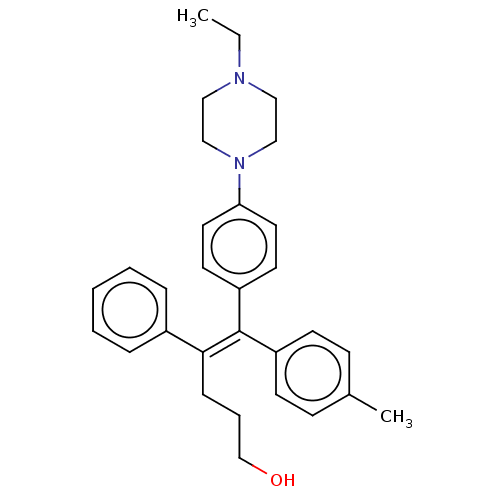
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 7.80nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPAMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:The thermal stability of MbtI in the absence and presence of inhibitors was measured using a fluorescence-based thermal shift assay.More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 5.83E+5nMAssay Description:Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPAMore data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant biotinylated KRAS G12D mutant (unknown origin) assessed as inhibition of SOS-catalyzed nucleotide exchange preincubated for...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inverse agonist activity at ERRgamma (unknown origin) by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Binding affinity to GST-tagged ERRgamma LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Binding affinity to GST-tagged ERRgamma LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair
TargetEstrogen-related receptor gamma(Homo sapiens (Human))
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Daegu-Gyeongbuk Medical Innovation Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at CMX-gal4-fused ERRgamma (unknown origin) transfected in human AD293 cells co-expressing CMV-beta galactosidase assessed a...More data for this Ligand-Target Pair

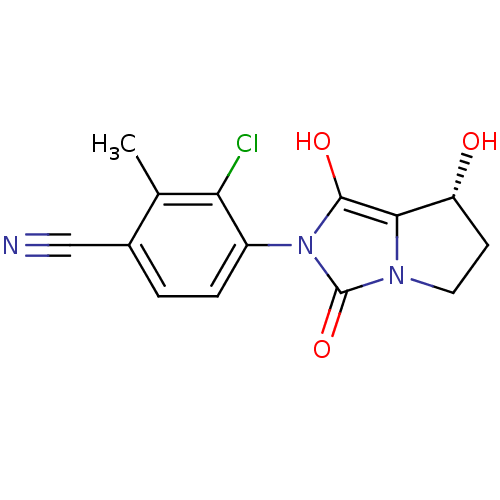

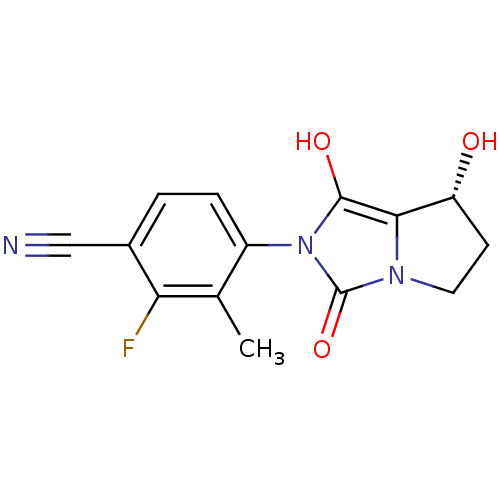


 3D Structure (crystal)
3D Structure (crystal)