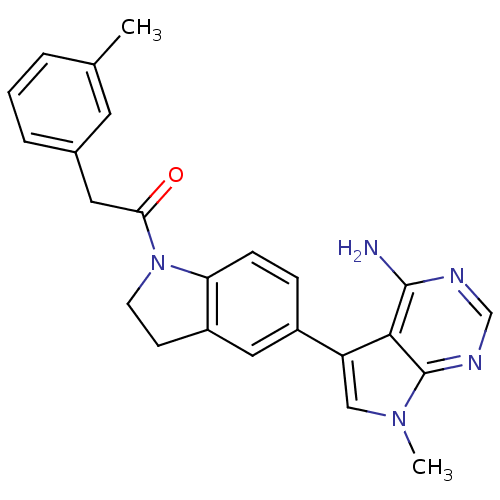
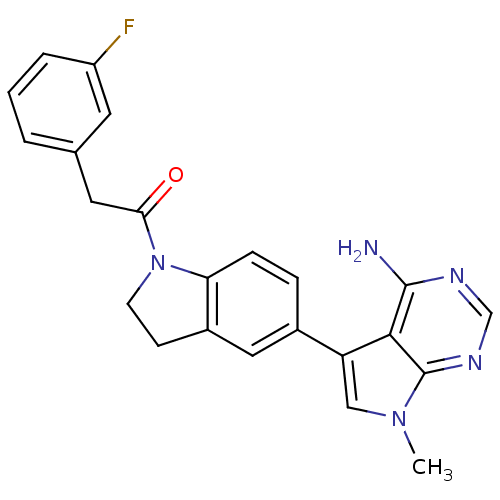
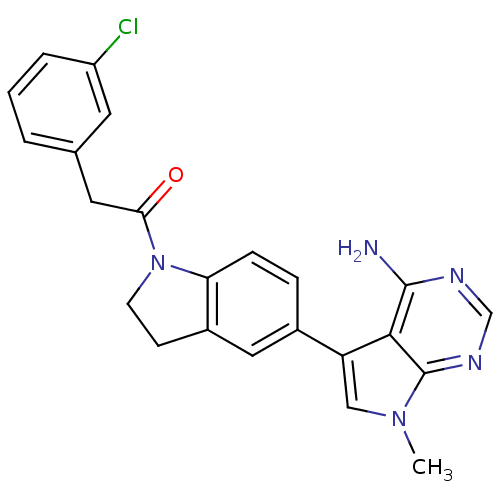
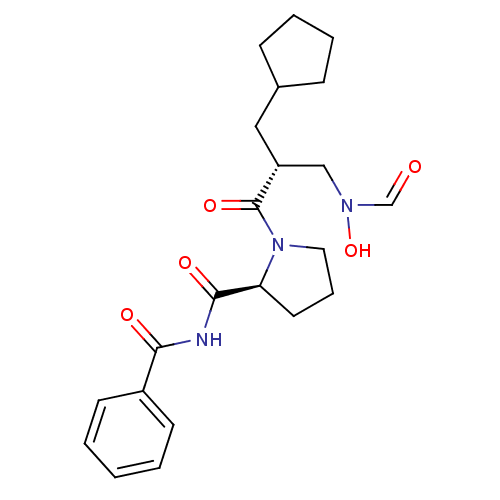
Affinity DataKi: 0.180nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.360nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 0.840nMAssay Description:Binding affinity to human D2RMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity to human D2RMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 1.75nM ΔG°: -49.5kJ/molepH: 7.4 T: 2°CAssay Description:The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Binding affinity to human D2RMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Binding affinity to human D2RMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Binding affinity to human D2RMore data for this Ligand-Target Pair
Affinity DataKi: 5.14nM ΔG°: -46.5kJ/molepH: 8.0 T: 2°CAssay Description:Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare).More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H] spiperone from human D2 dopamine receptor expressed in monkey caudate-putamen membranesMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 97nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 106nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 131nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 146nM ΔG°: -38.6kJ/molepH: 7.4 T: 2°CAssay Description:The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst...More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 290nM ΔG°: -36.7kJ/molepH: 8.0 T: 2°CAssay Description:Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare).More data for this Ligand-Target Pair
Affinity DataKi: 301nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nM ΔG°: -34.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ...More data for this Ligand-Target Pair
Affinity DataKi: 1.81E+3nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.89E+3nM ΔG°: -32.3kJ/molepH: 7.4 T: 2°CAssay Description:The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst...More data for this Ligand-Target Pair
Affinity DataKi: 2.11E+3nMAssay Description:Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysisMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B(Marthasterias glacialis (starfish))
University of Newcastle
University of Newcastle
Affinity DataKi: 2.50E+3nM ΔG°: -32.5kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B(Marthasterias glacialis (starfish))
University of Newcastle
University of Newcastle
Affinity DataKi: 5.00E+3nM ΔG°: -30.8kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nM ΔG°: -28.6kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylationMore data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylationMore data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylationMore data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylationMore data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylationMore data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.260nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair
TargetPeptide deformylase(Streptococcus pneumoniae serotype 4 (strain ATCC B...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.280nMAssay Description:Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass...More data for this Ligand-Target Pair

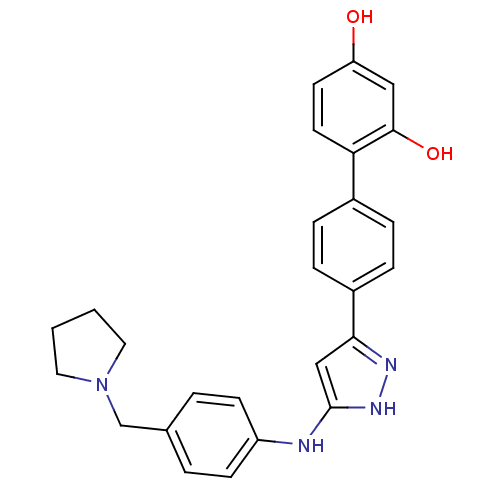
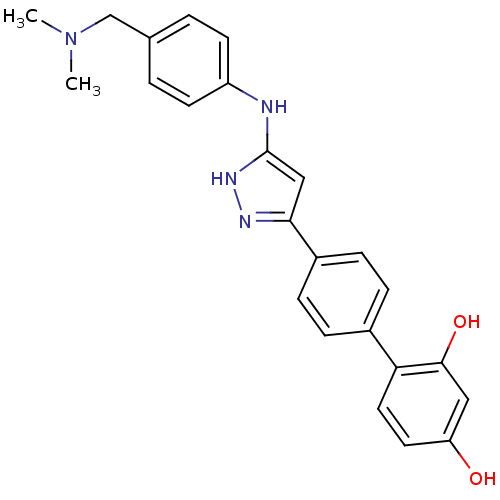
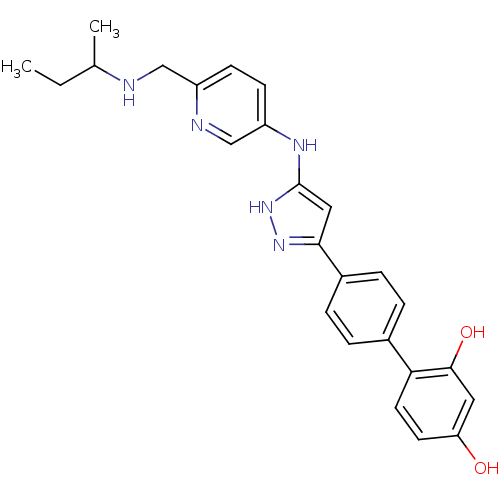
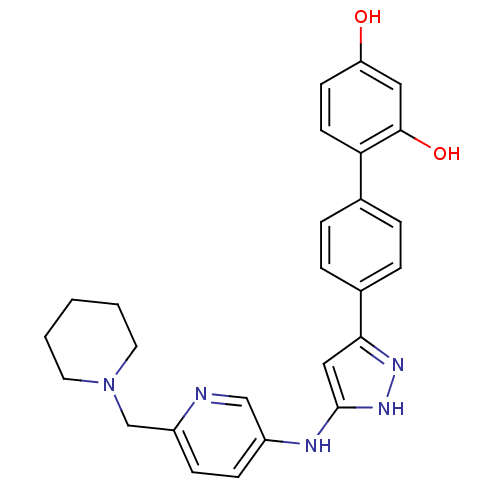


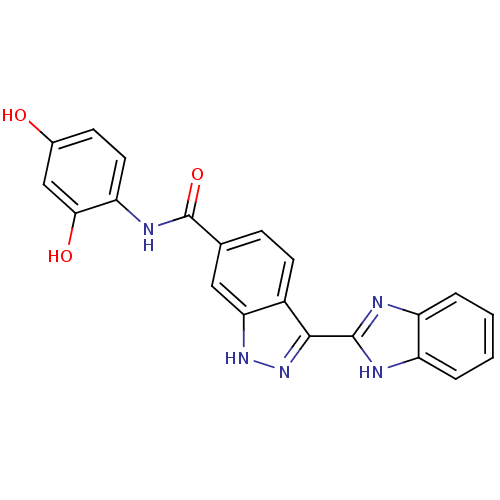
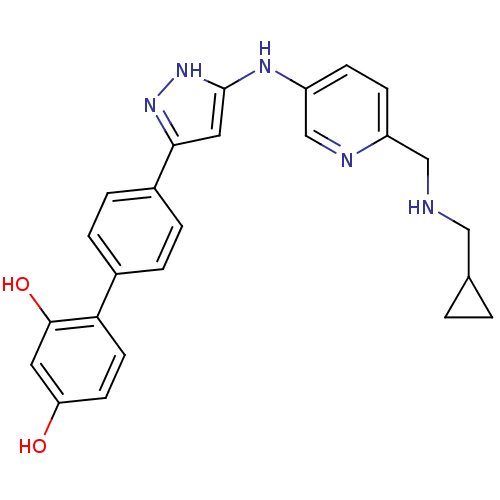
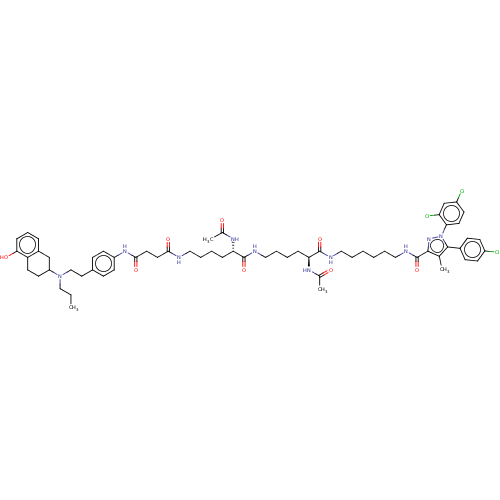
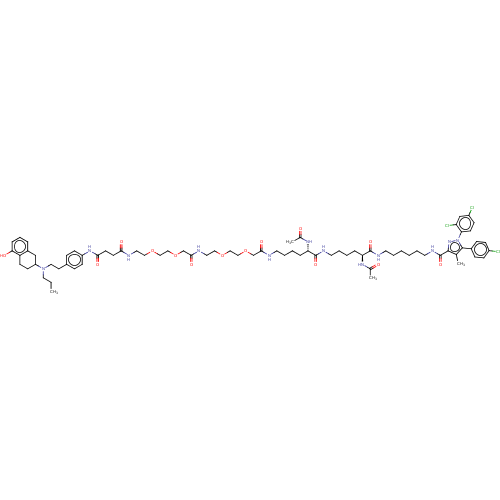
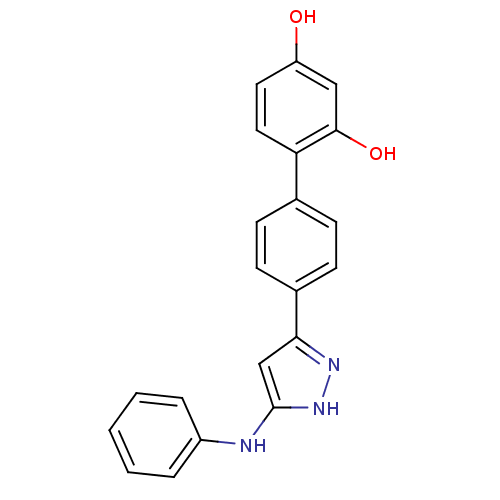
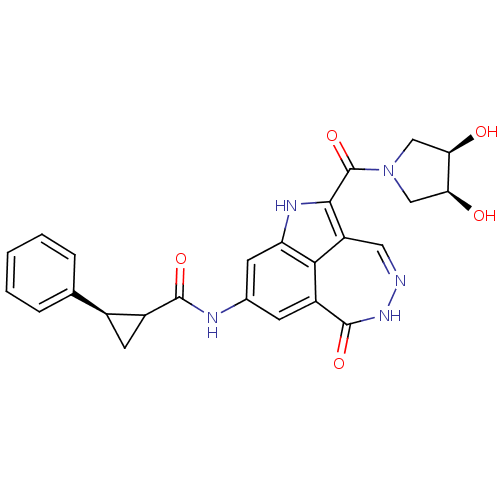
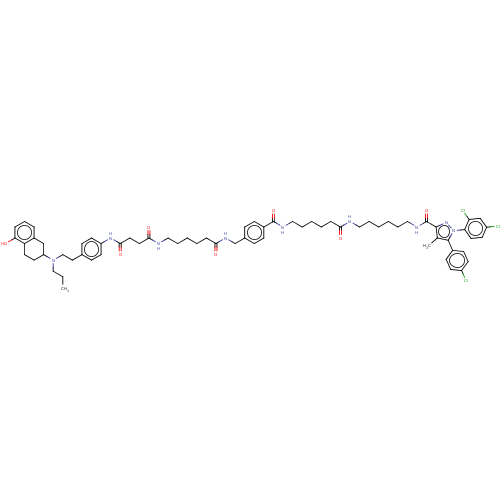

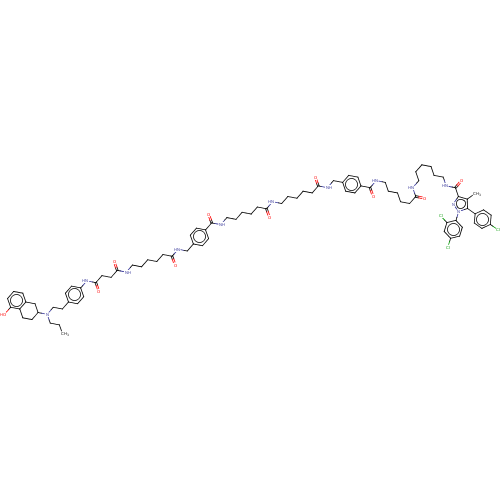
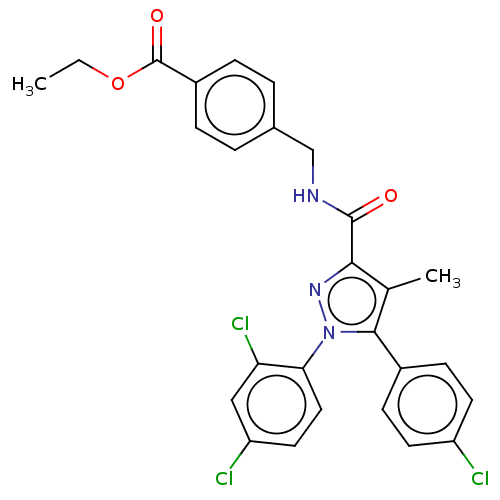
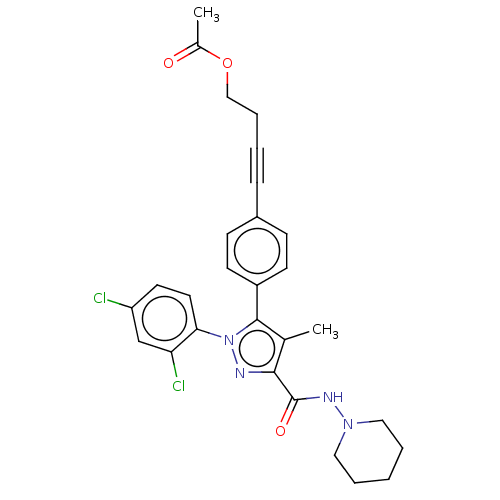
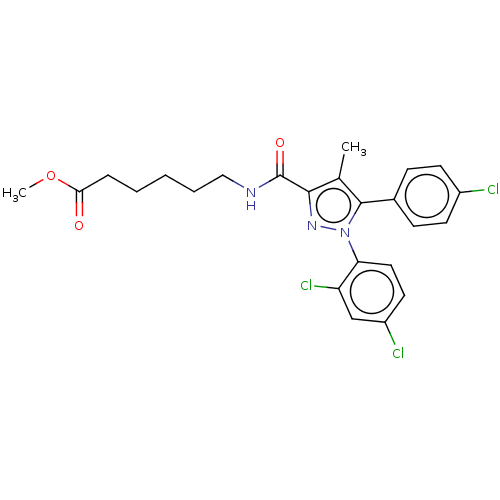
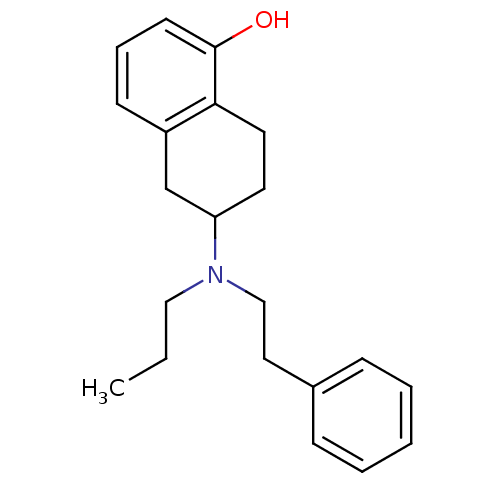
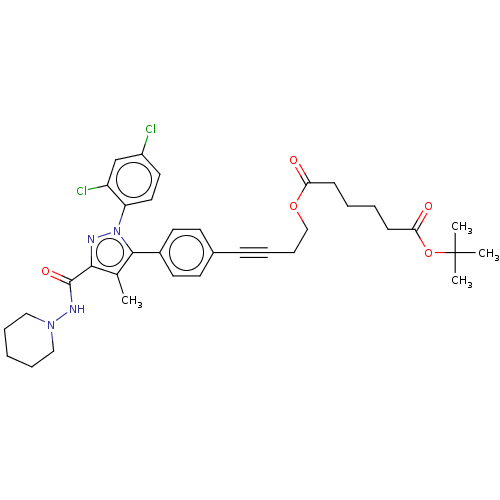
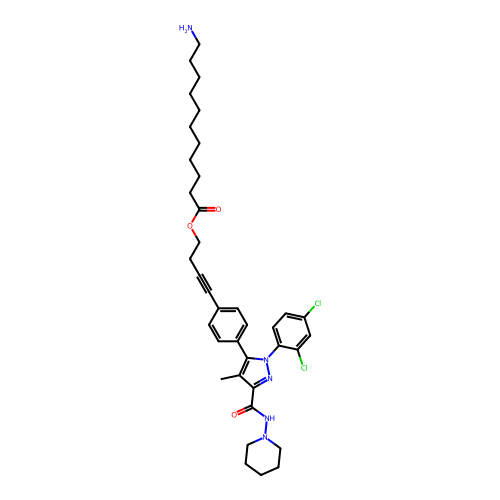
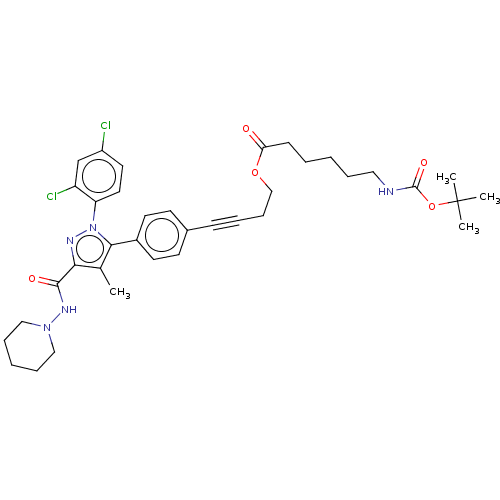
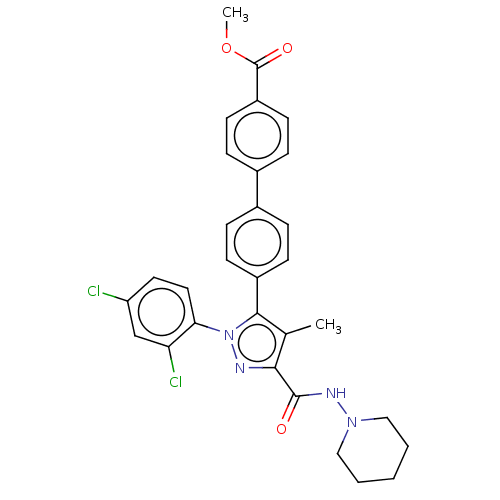
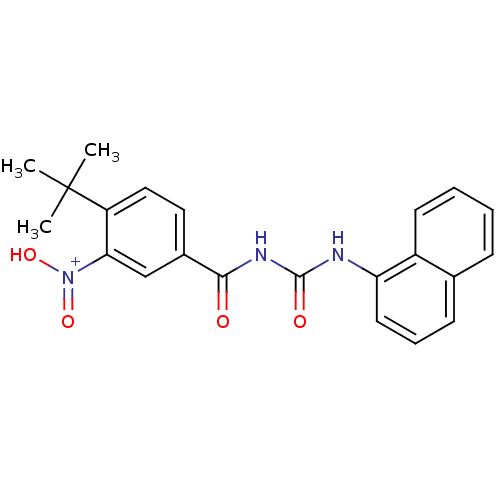

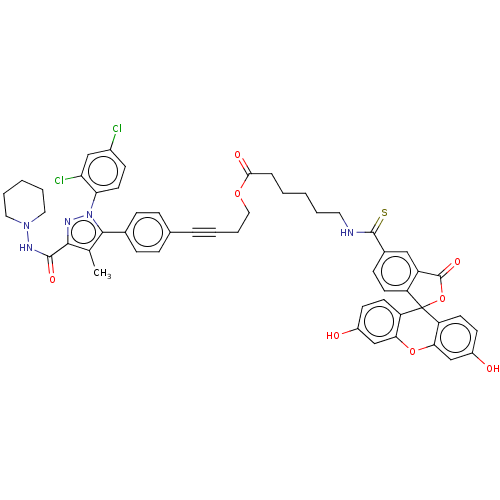
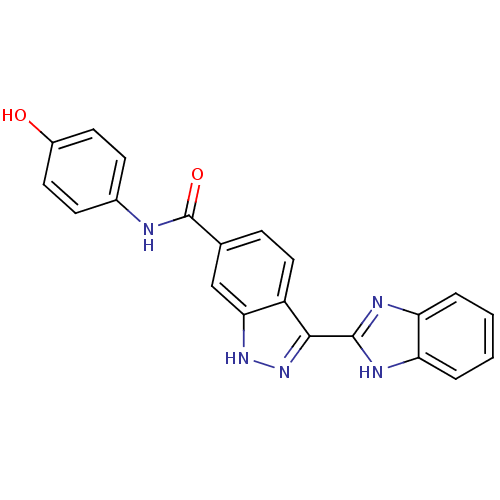
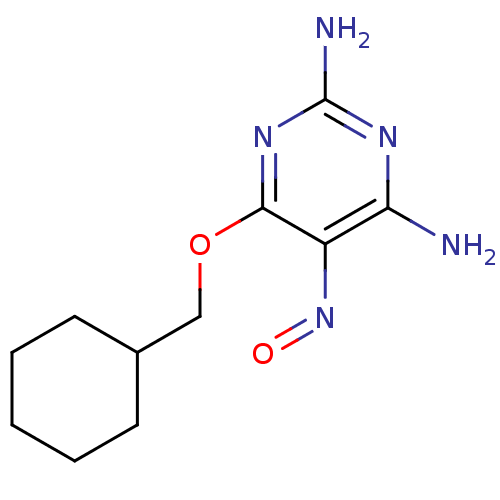
 3D Structure (crystal)
3D Structure (crystal)