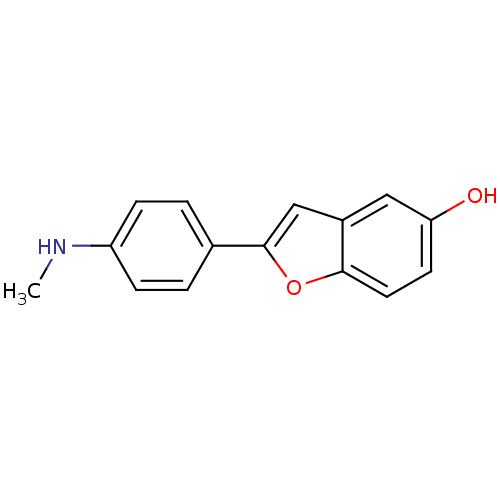
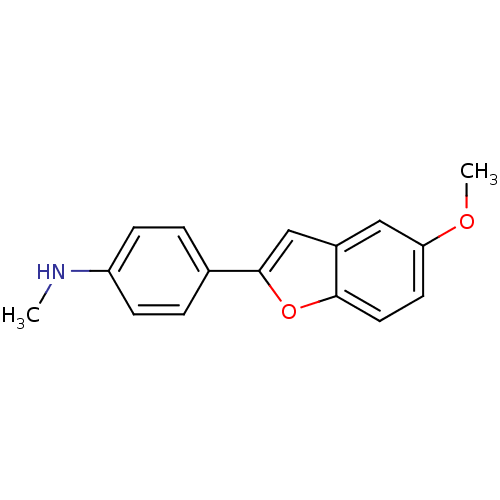
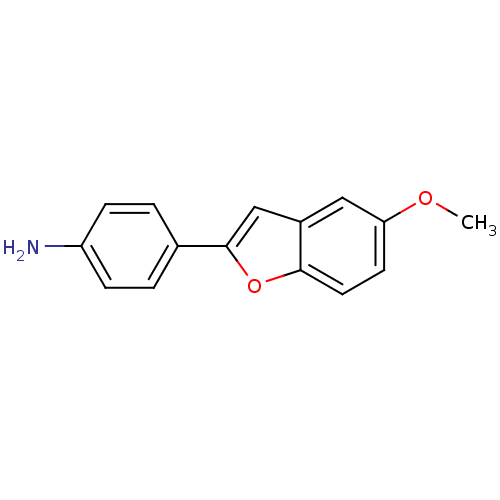
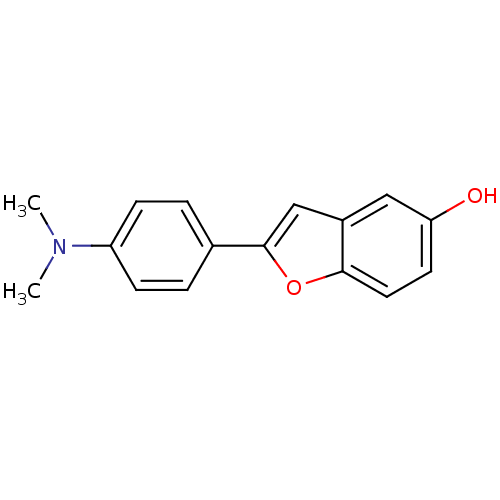
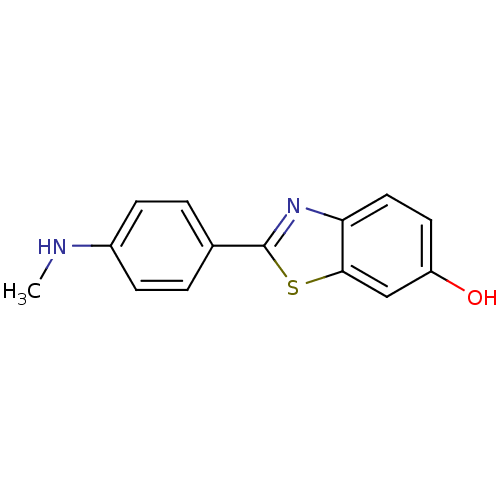
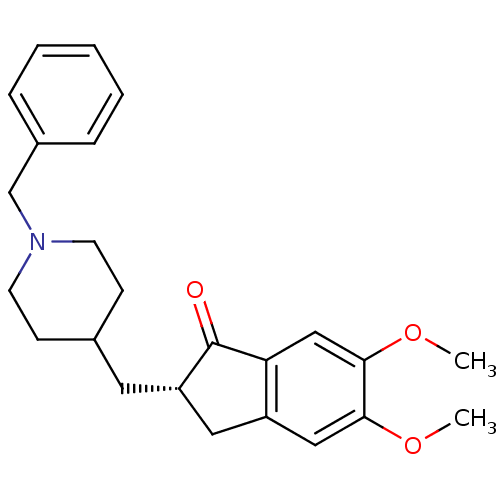
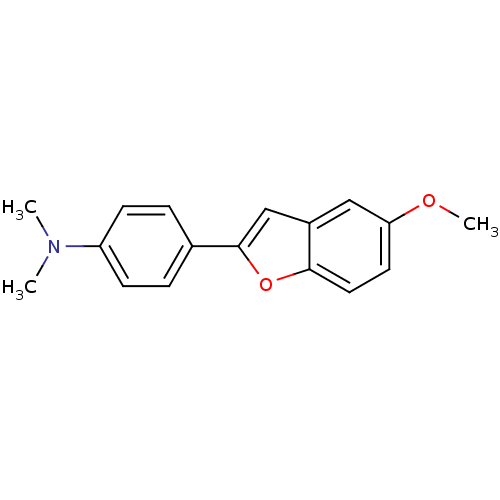
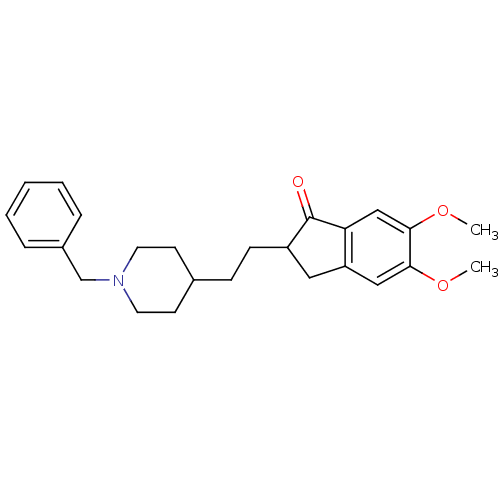
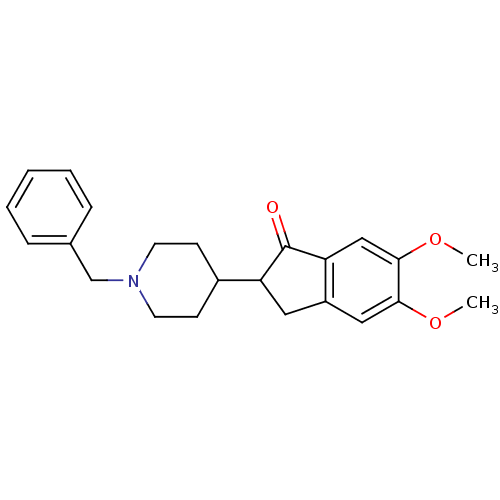
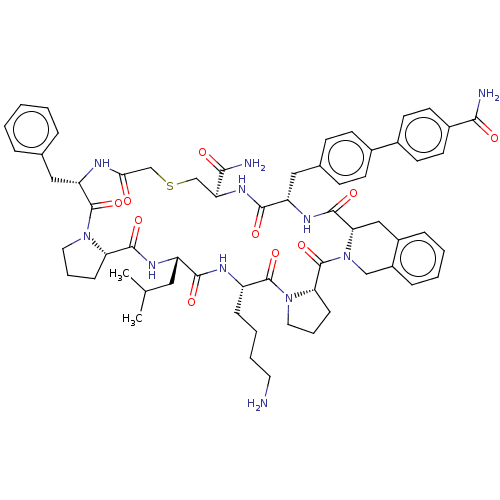
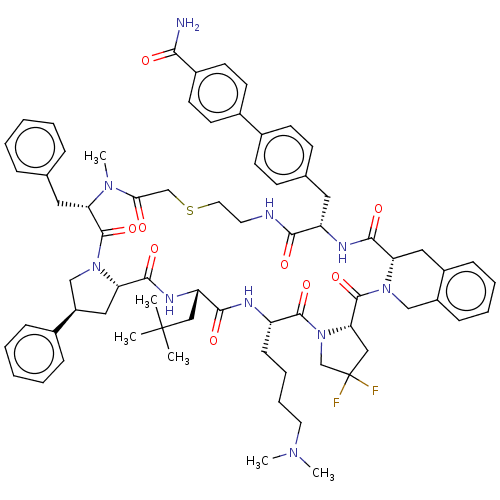
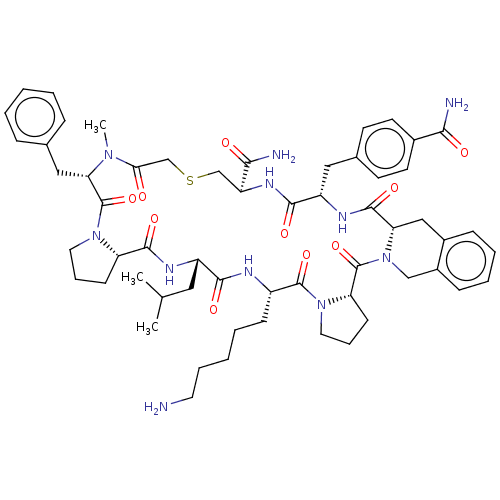
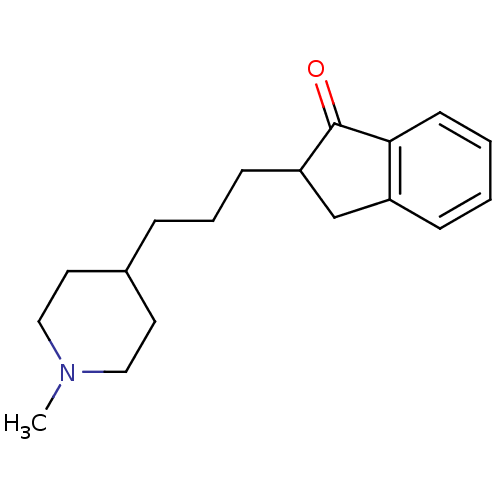
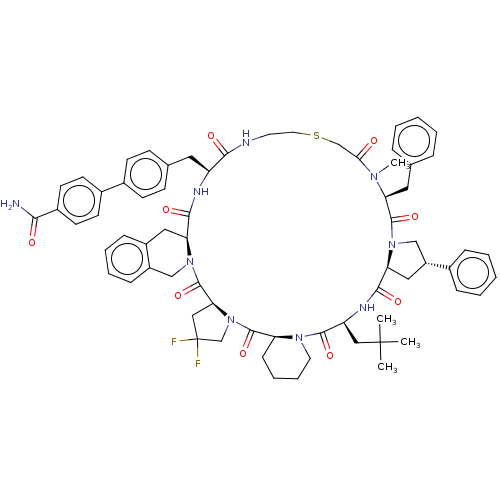
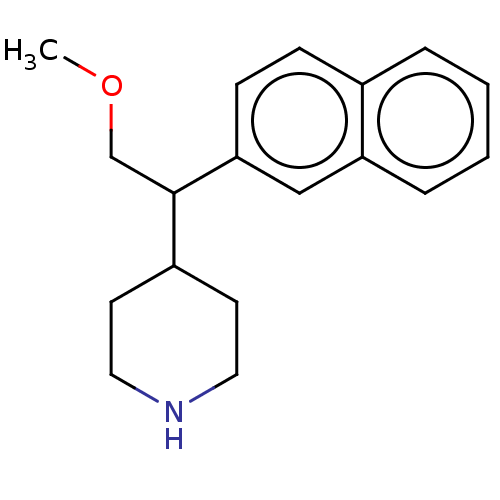
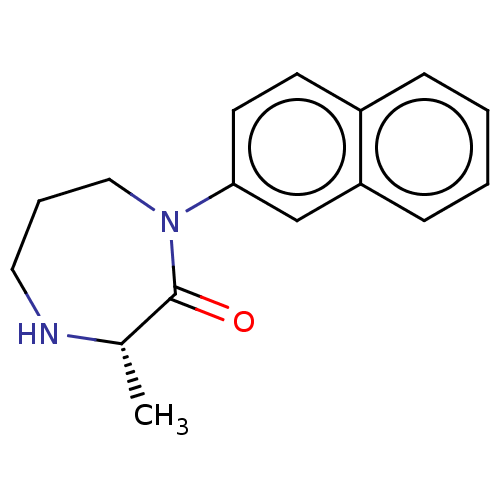
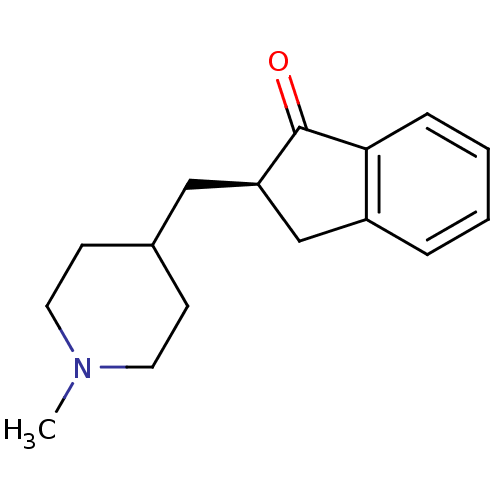
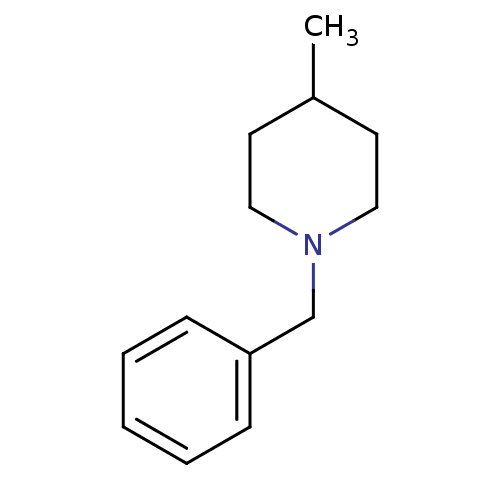
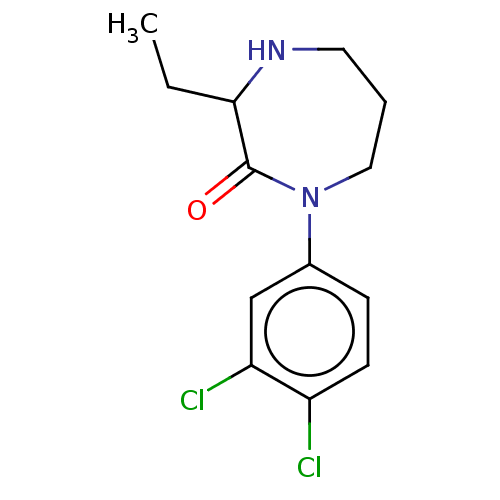
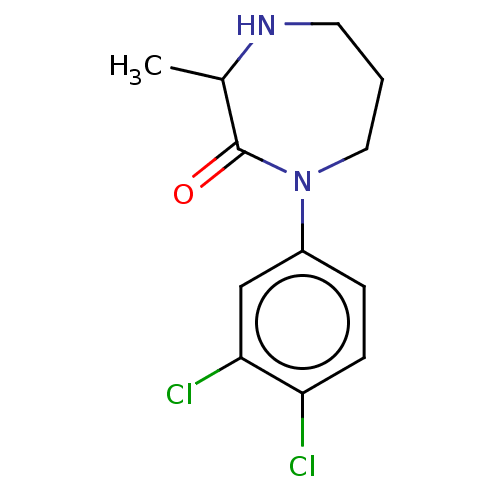
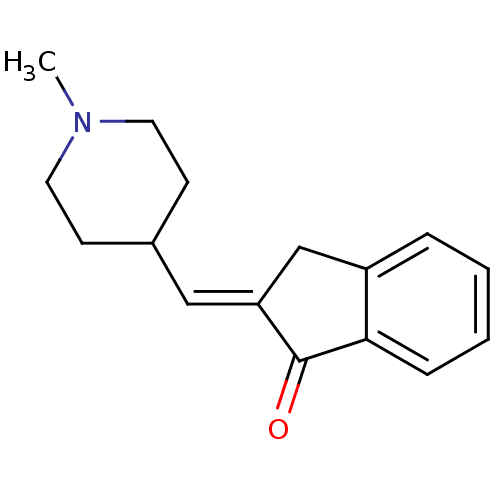
Affinity DataKi: 0.700nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity to beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 9.5nMAssay Description:Inhibitory activity against AcetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 9.5nMAssay Description:Inhibitory activity against AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataKi: 11.5nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [125I]IMPY from beta amyloid in human corpse AD brainMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 17.5nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Inhibitory activity against AcetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 37.6nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 54nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 54nMAssay Description:Inhibitory activity against AcetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
Eisai
Curated by ChEMBL
Eisai
Curated by ChEMBL
Affinity DataKi: 122nMAssay Description:Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1)More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.930nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.950nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Displacement of [3H]-hydroxytryptamine from human SERT expressed in CHO cells by TopCounting analysisMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Kyorin Pharmaceutical
Curated by ChEMBL
Kyorin Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate after 60 mins by OPA reagent based fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in CHO cells after 45 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Displacement of [3H]-hydroxytryptamine from human SERT expressed in CHO cells by TopCounting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Displacement of [3H]-hydroxytryptamine from human SERT expressed in CHO cells by TopCounting analysisMore data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in CHO cells after 45 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in CHO cells after 45 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40nMAssay Description:Inhibition of acetylcholinesterase activityMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Displacement of [3H]-norepinephrine from human NET expressed in CHO cells by TopCounting analysisMore data for this Ligand-Target Pair