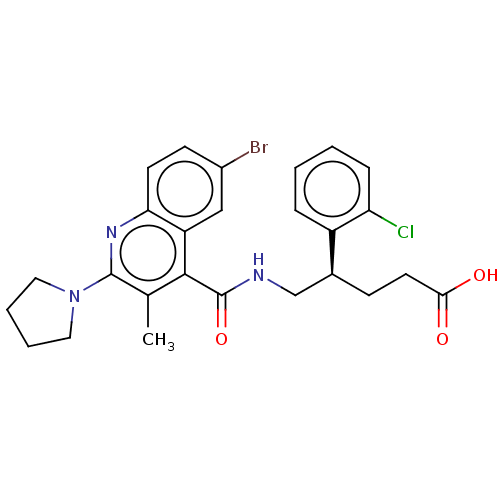
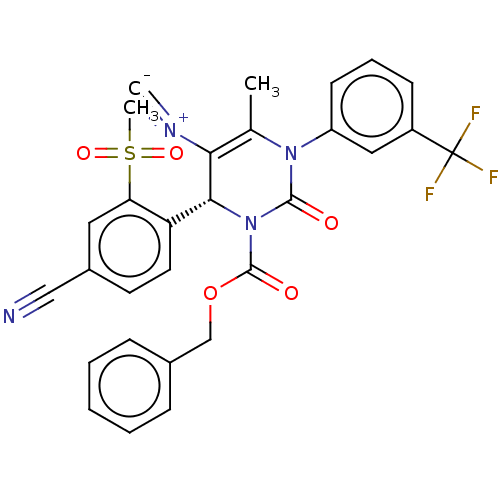
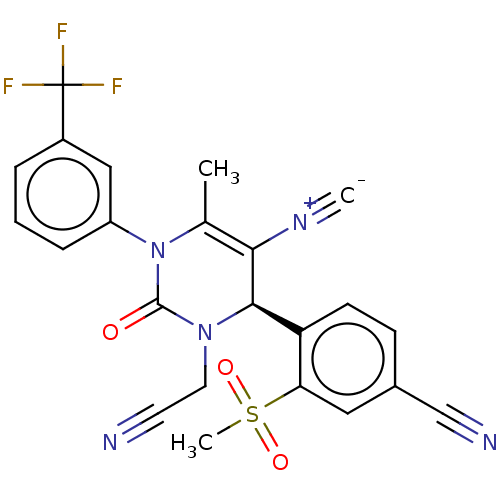
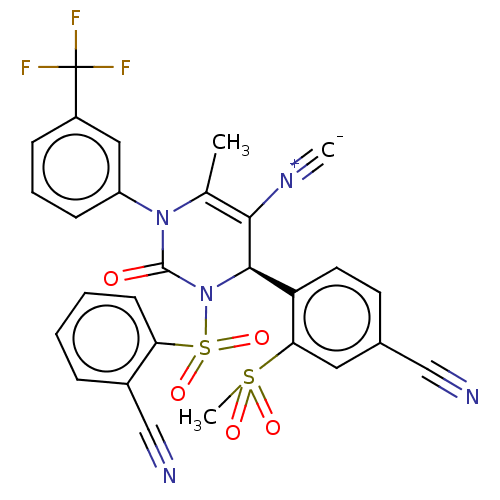
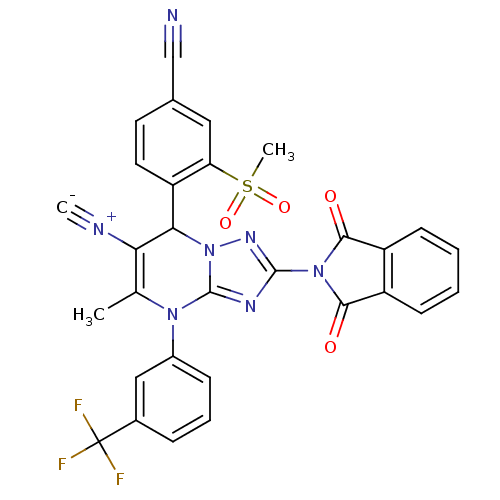
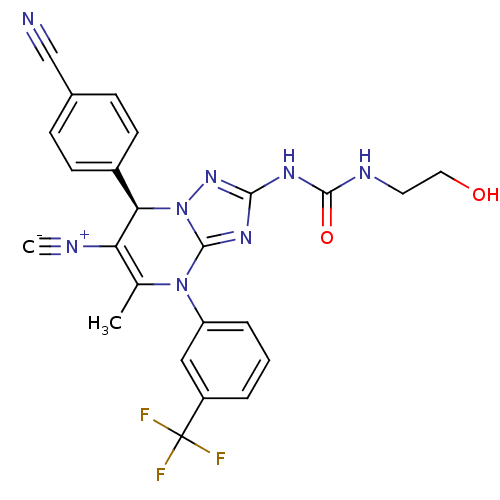
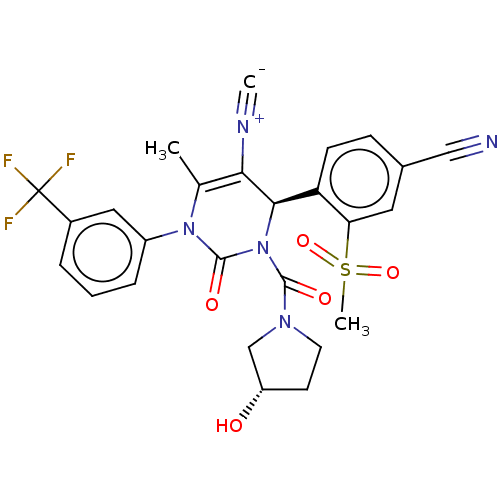
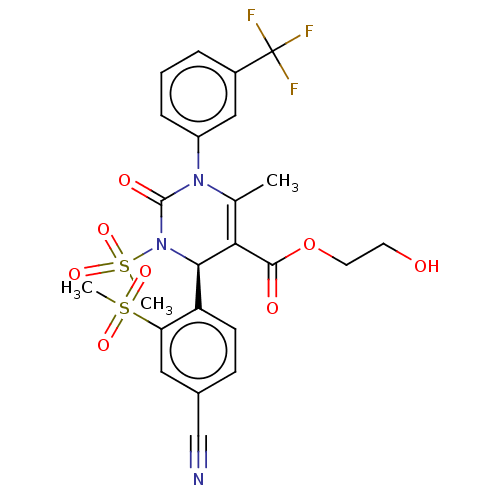
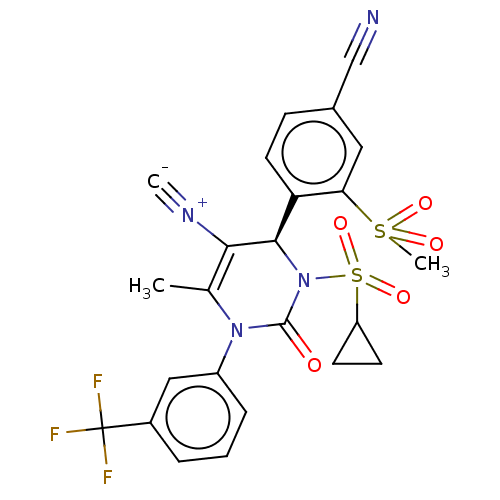
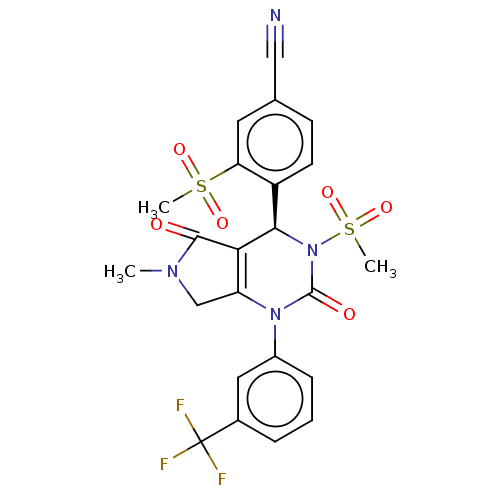
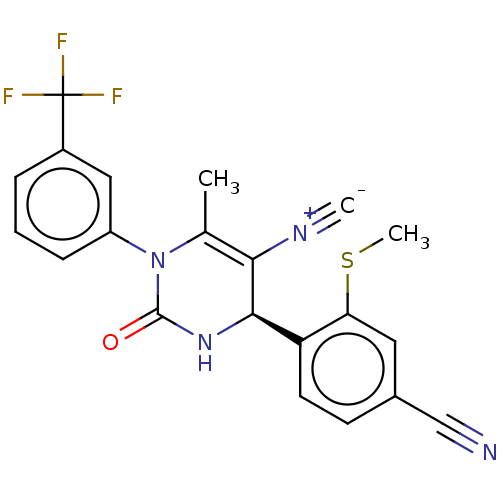
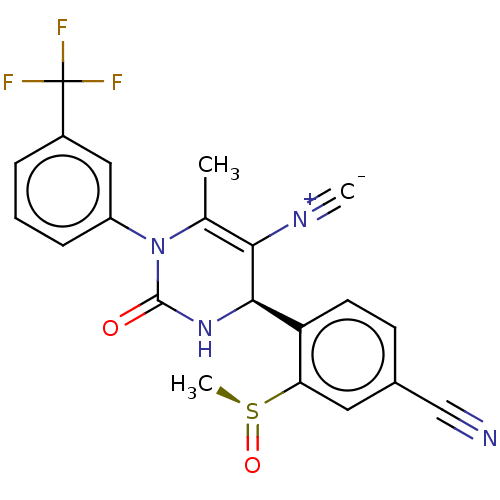
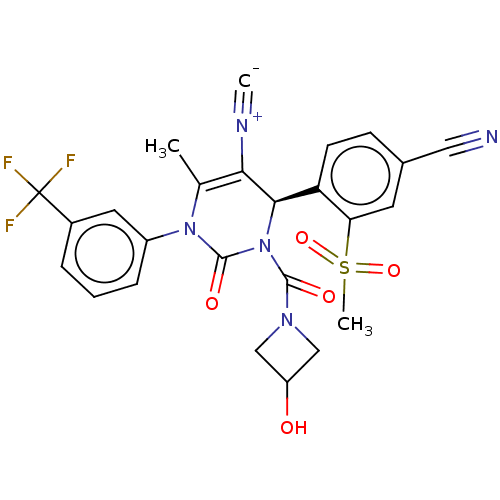
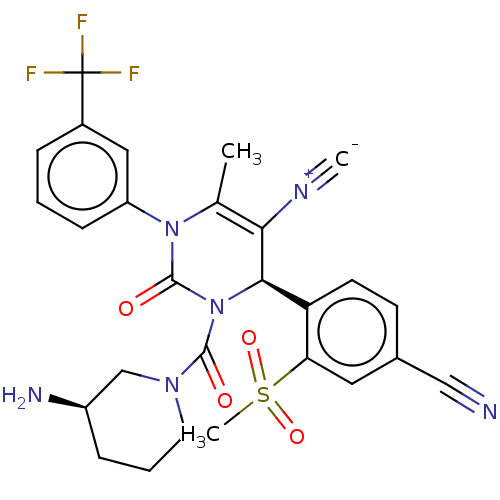
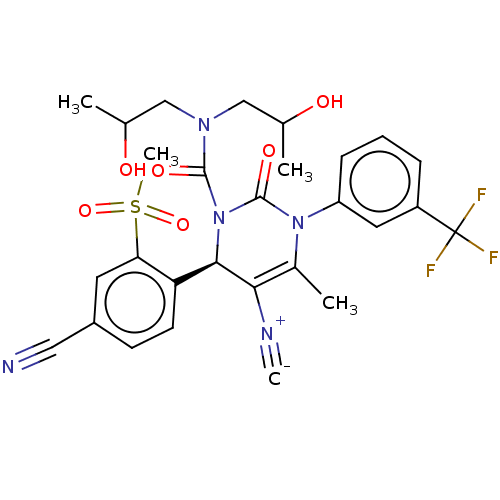
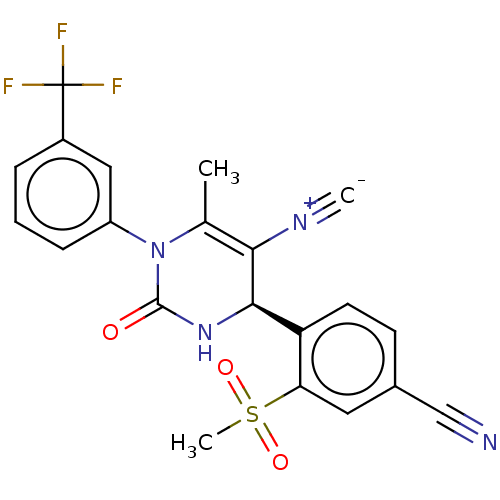
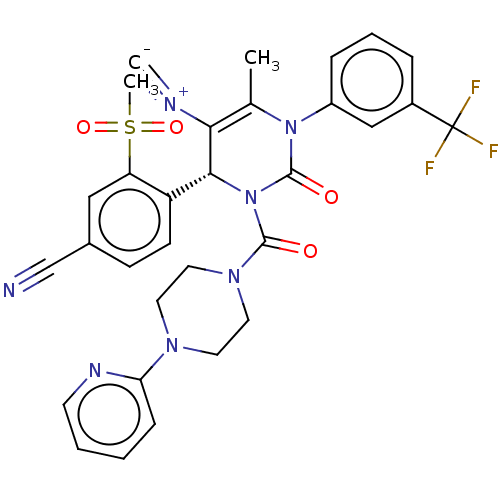
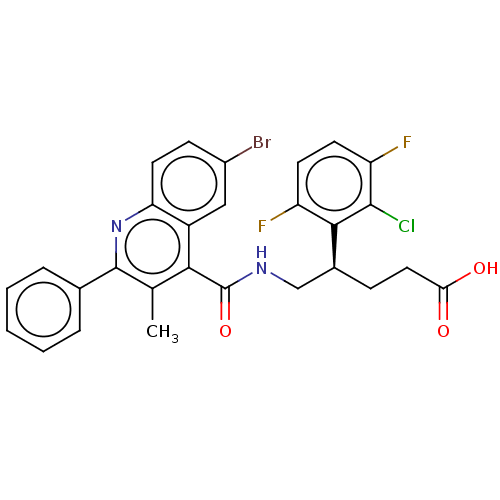
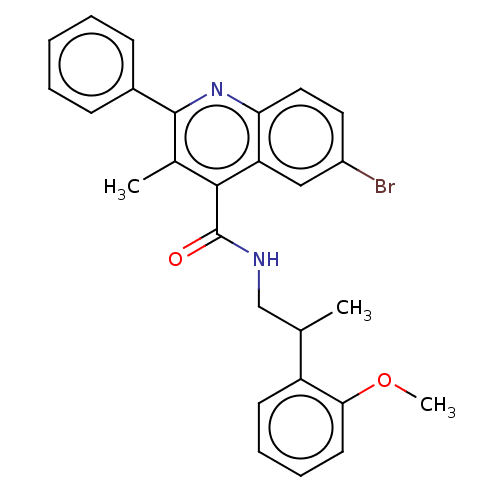
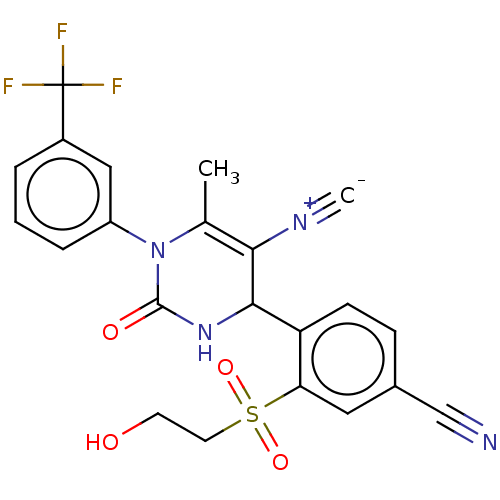
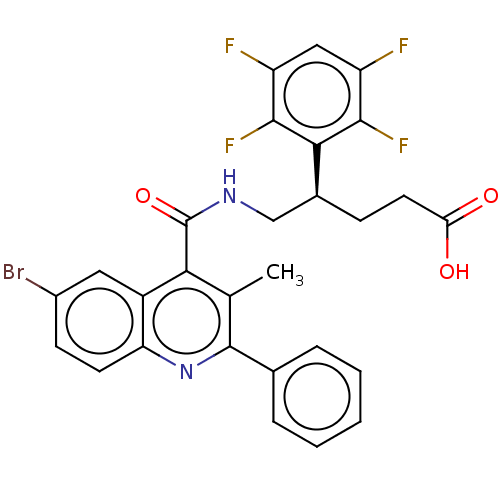
Affinity DataKi: 16nMAssay Description:Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati...More data for this Ligand-Target Pair
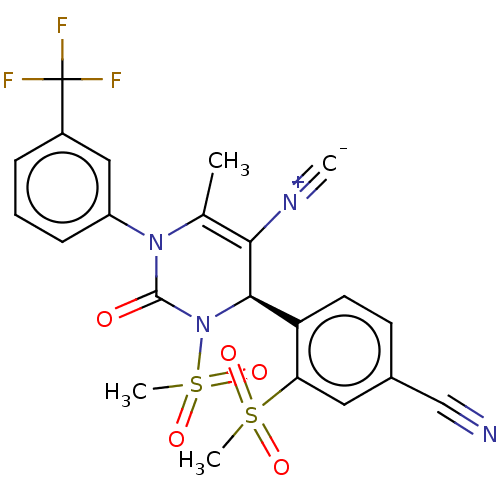
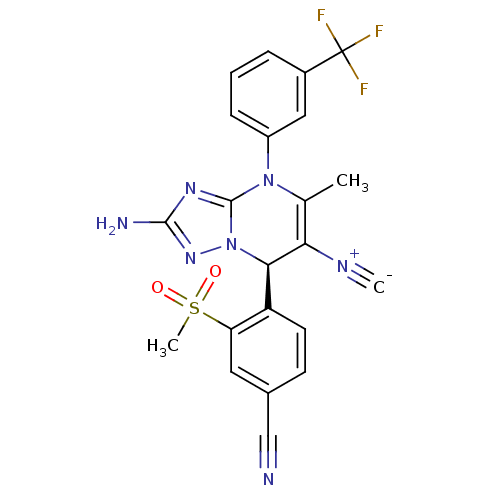
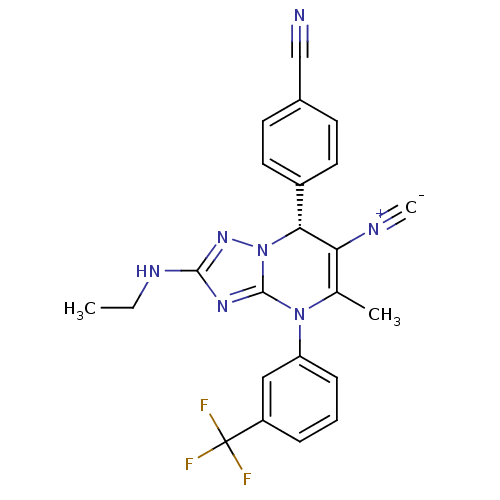
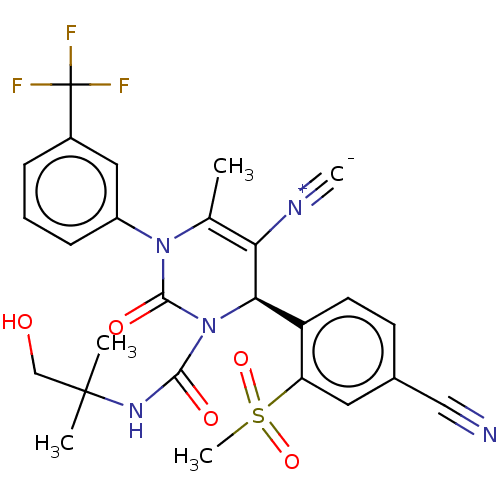
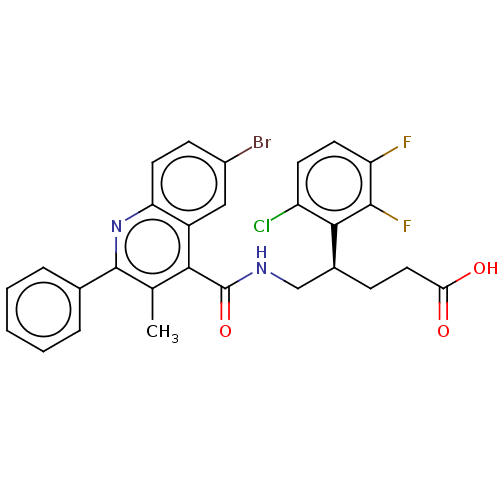
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
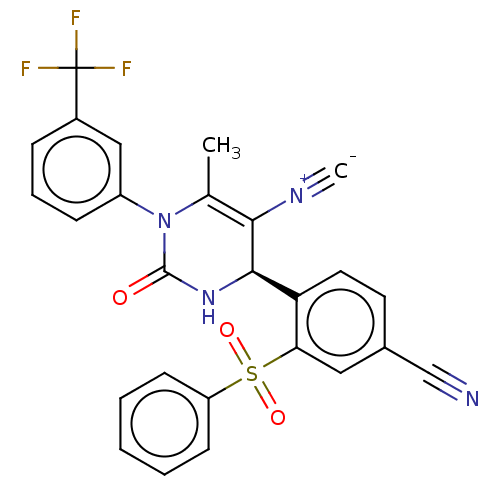
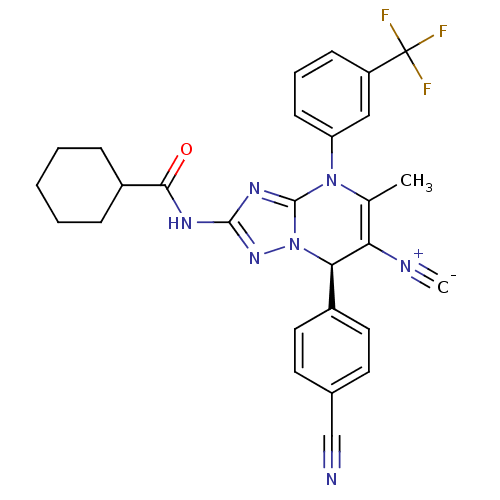
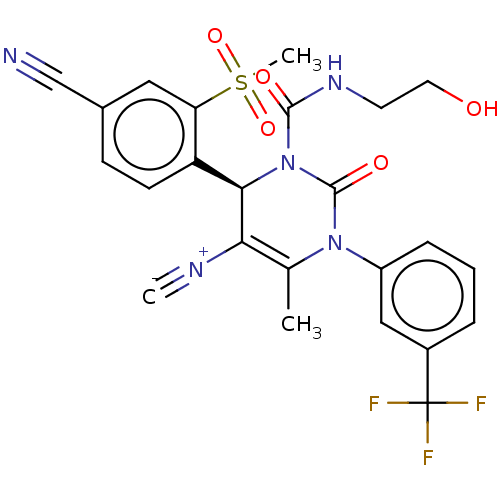
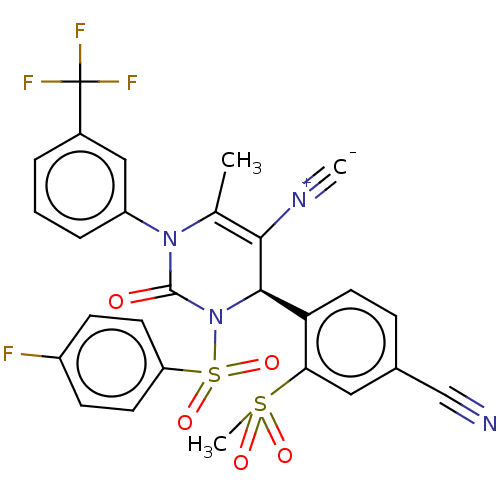
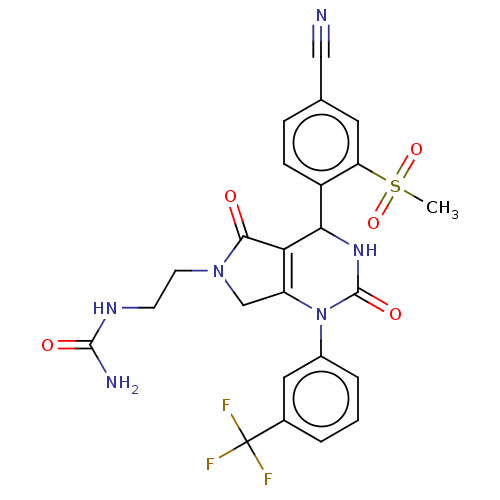
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
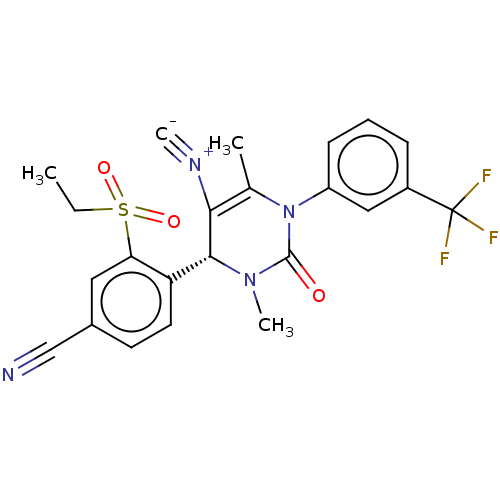
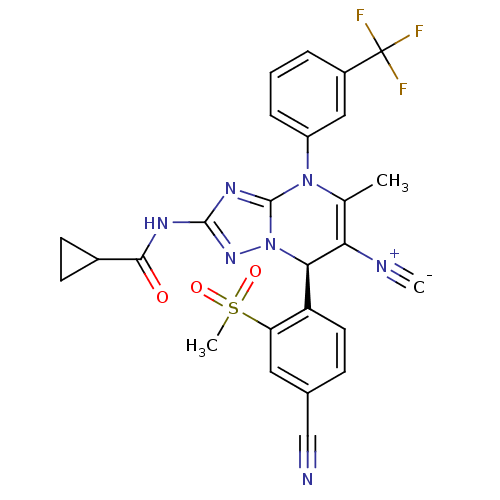
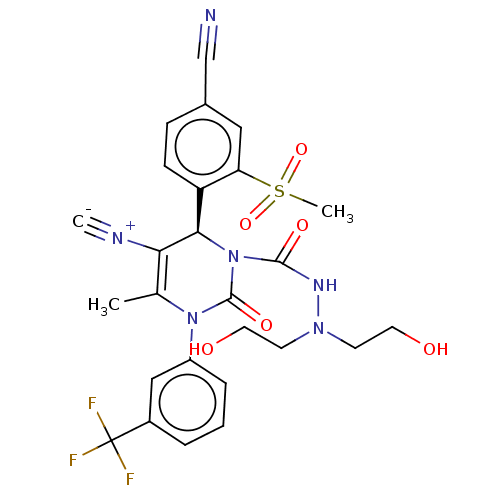
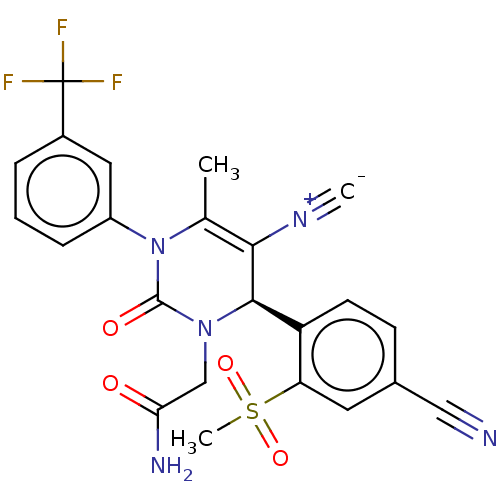
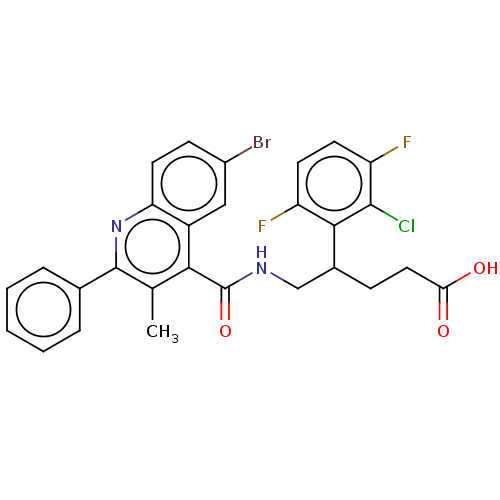
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Antagonist activity at human FPR expressed in human Chem-1 cells assessed as inhibition of PGF2alpha-induced calcium flux preincubated for 10 mins fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Antagonist activity at human FPR expressed in human Chem-1 cells assessed as inhibition of PGF2alpha-induced calcium flux preincubated for 10 mins fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.830nMAssay Description:Antagonist activity at human FPR expressed in human Chem-1 cells assessed as inhibition of PGF2alpha-induced calcium flux preincubated for 10 mins fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
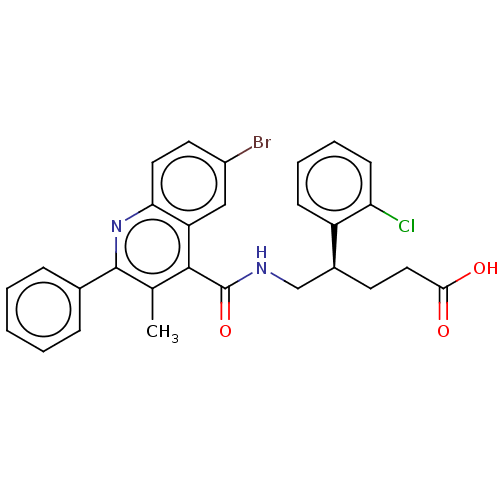
Affinity DataIC50: <1nMAssay Description:Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:For the characterization of test substances in respect of FP antagonism, PGF2α-induced calcium flux in FP-expressing CHEM1 cells (Millipore, HTS...More data for this Ligand-Target Pair