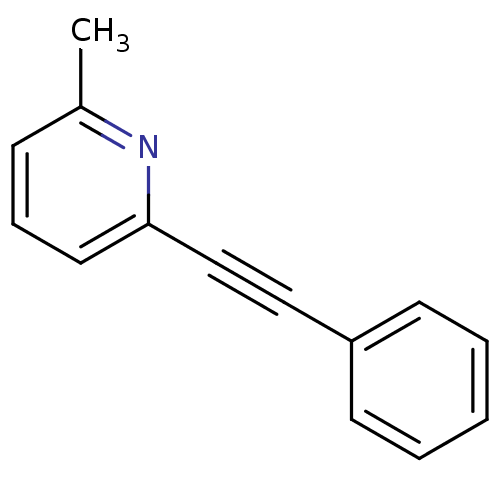
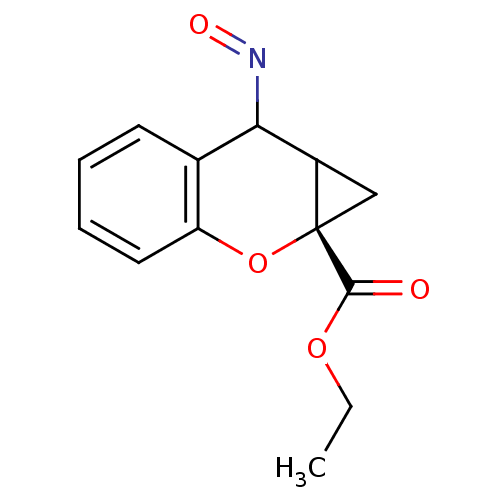
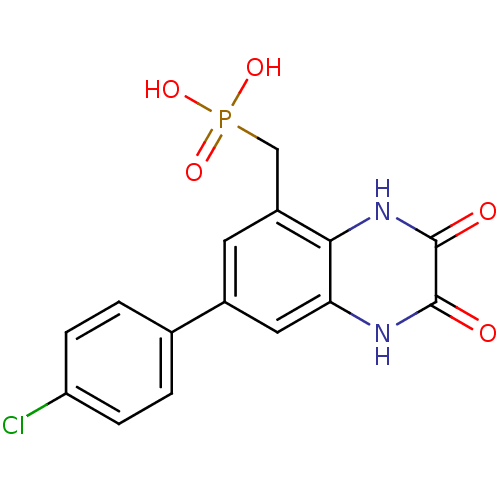
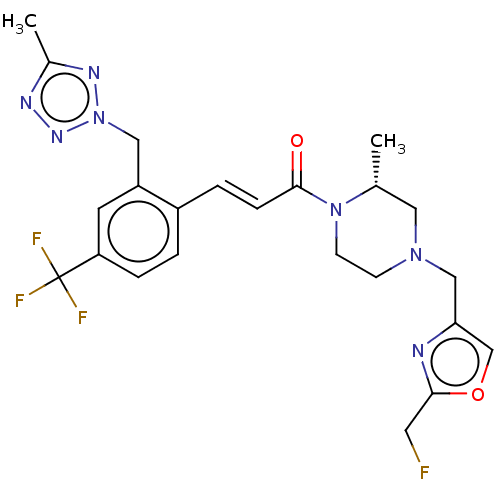
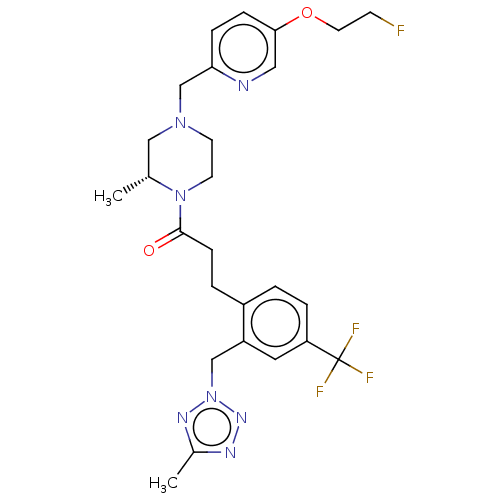
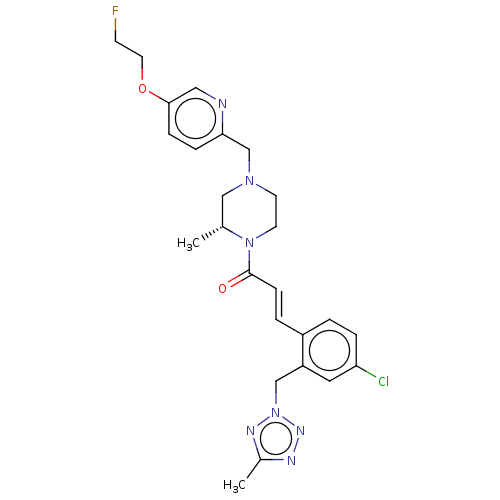
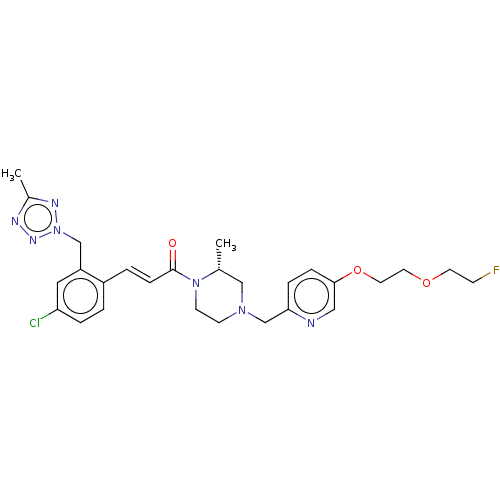
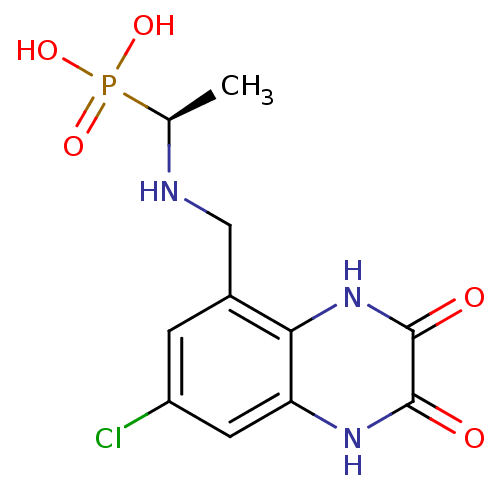
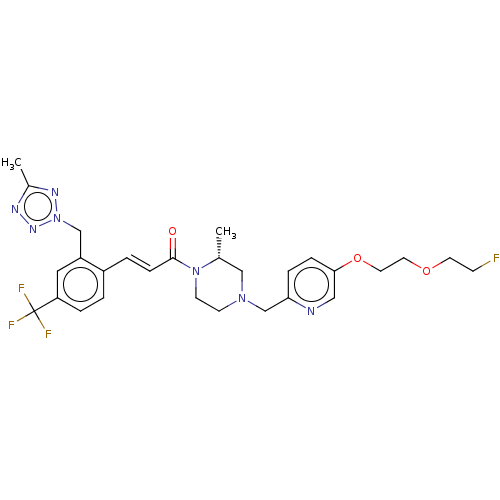
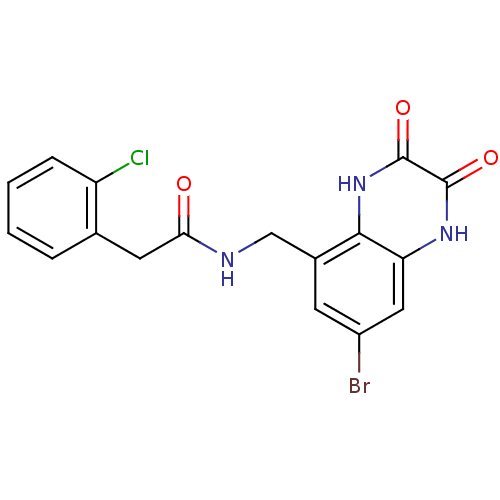
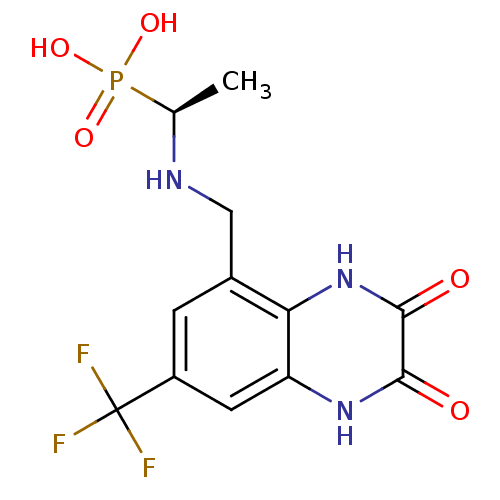
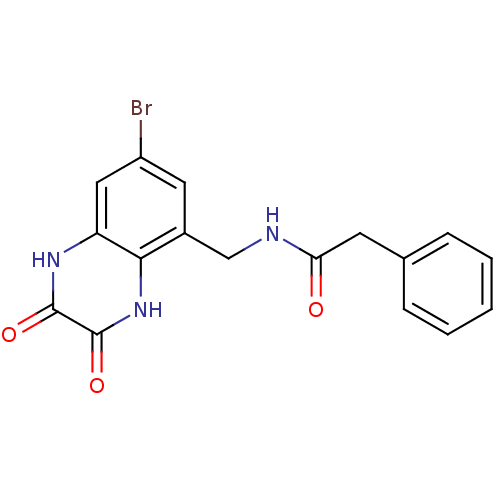
Affinity DataKi: 0.5nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
Affinity DataKi: 10nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
Affinity DataKi: 12nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 26nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 31nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 44nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2A heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of glutamate-induced calcium releaseMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of quisqualate-induced phosphoinositol accumulationMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Hospital Zurich
Curated by ChEMBL
University Hospital Zurich
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Hospital Zurich
Curated by ChEMBL
University Hospital Zurich
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Hospital Zurich
Curated by ChEMBL
University Hospital Zurich
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Hospital Zurich
Curated by ChEMBL
University Hospital Zurich
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Hospital Zurich
Curated by ChEMBL
University Hospital Zurich
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair

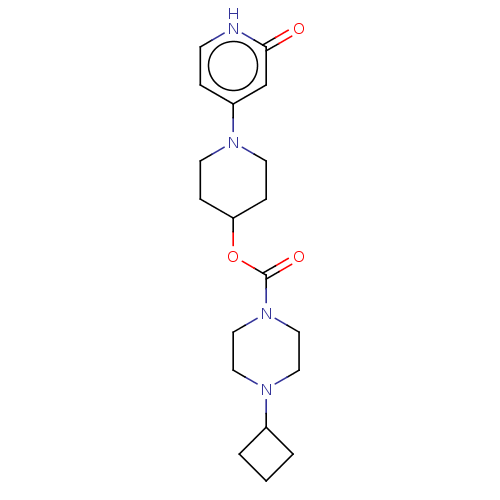
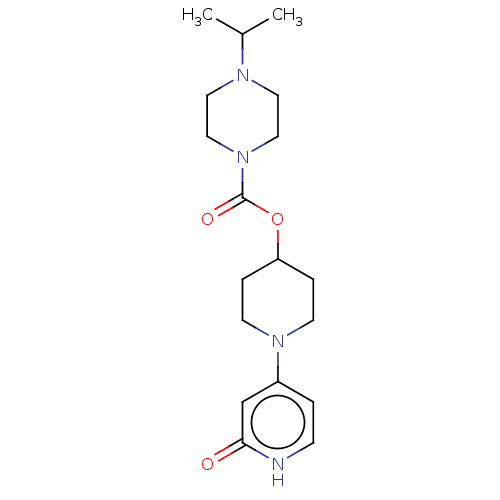
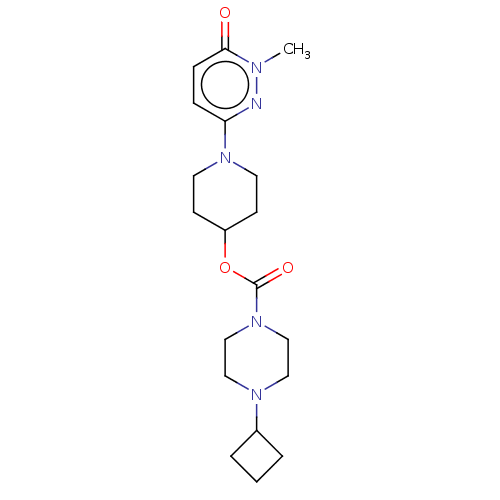
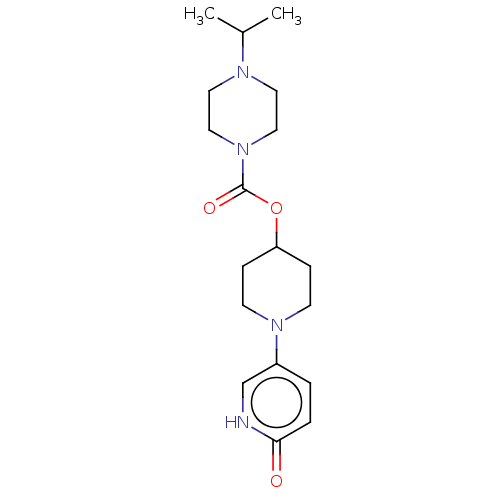
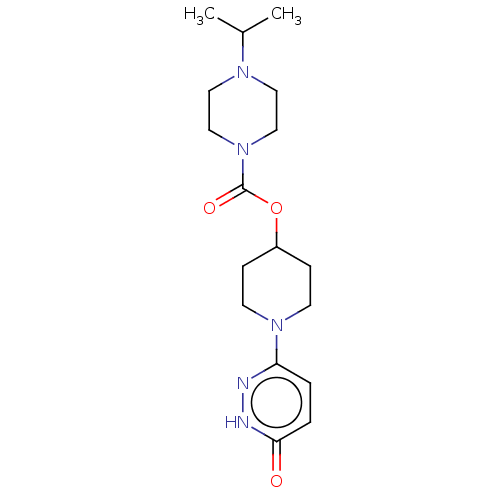



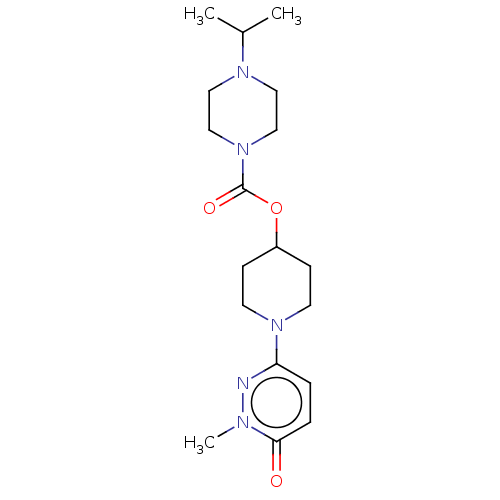
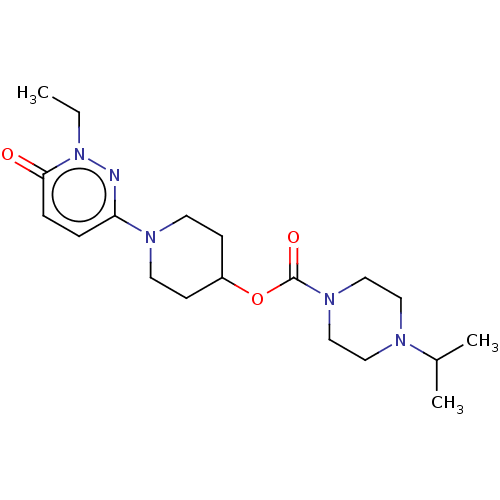
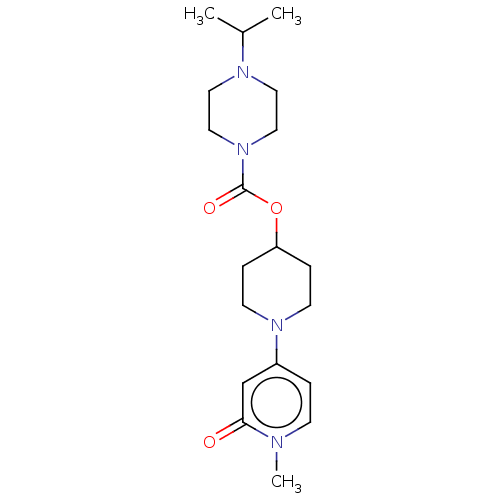

 3D Structure (crystal)
3D Structure (crystal)