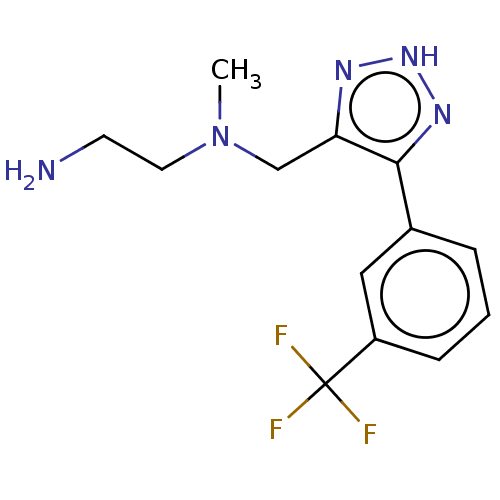
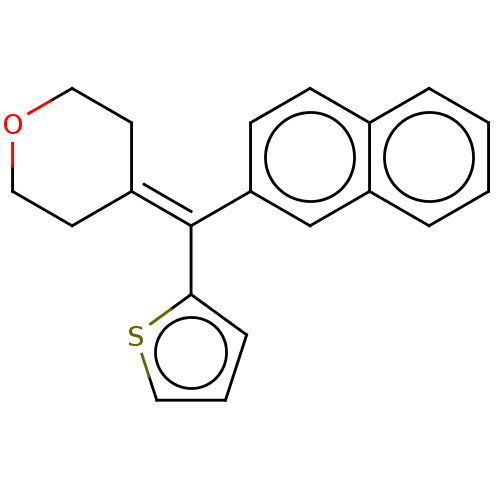
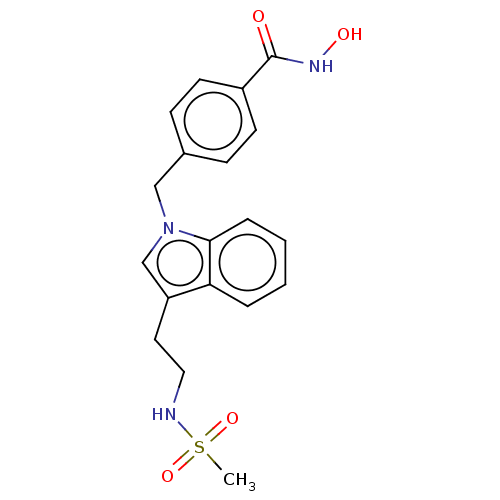
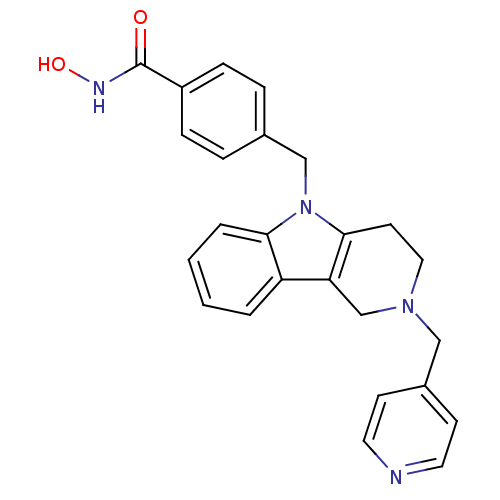
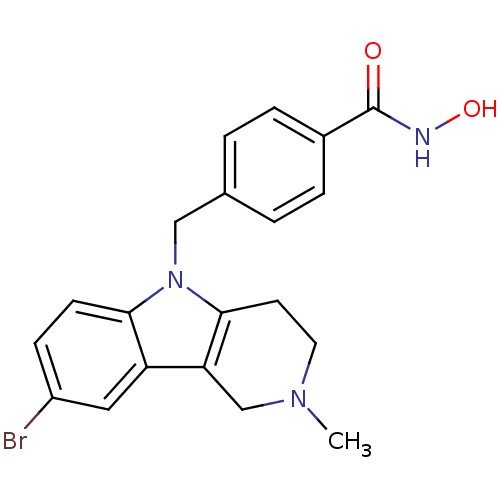
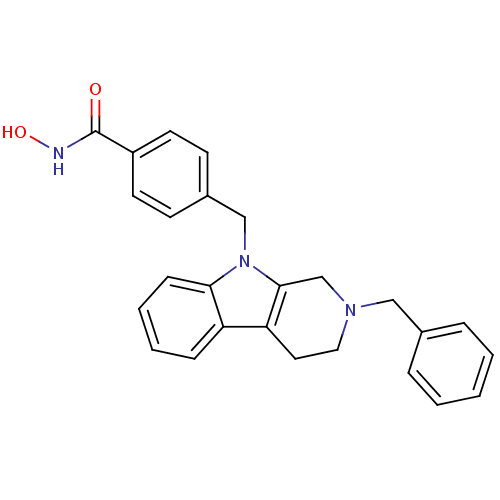
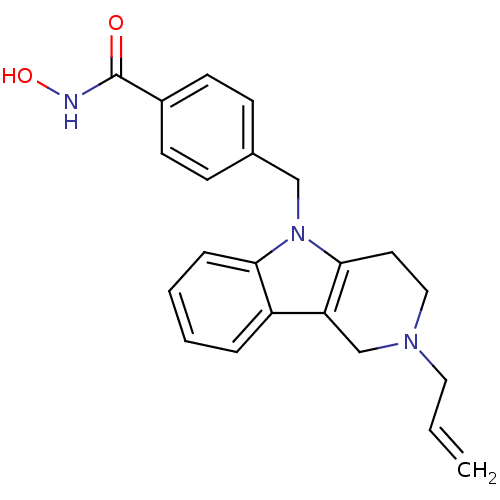
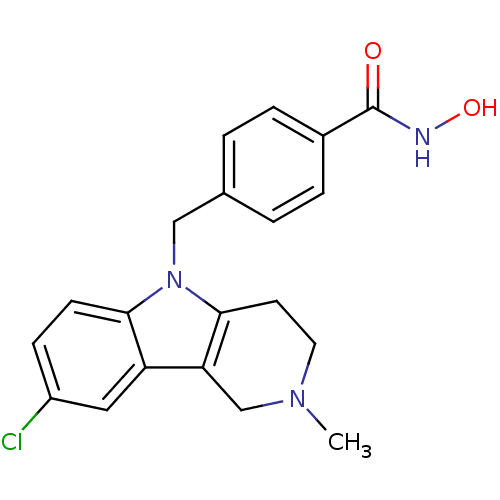
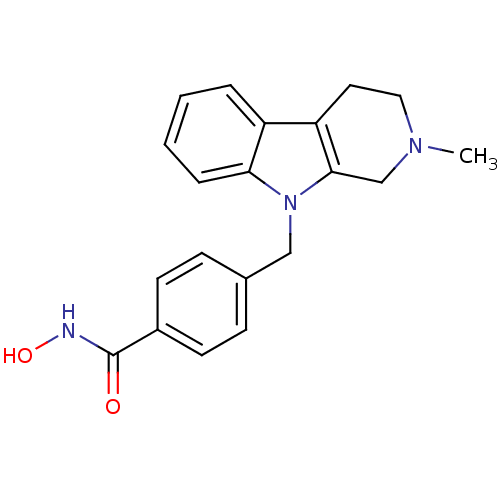
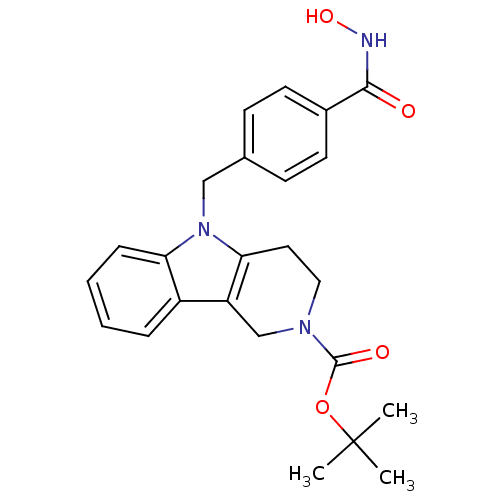
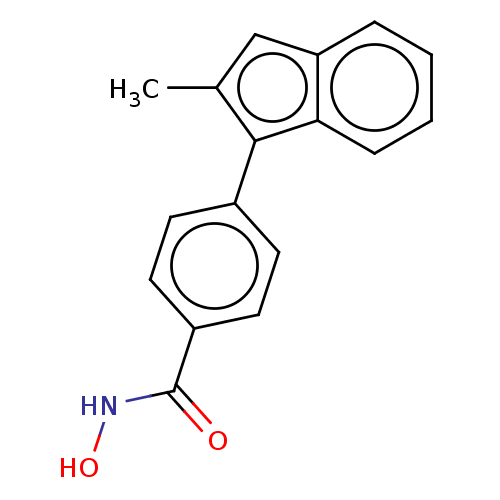
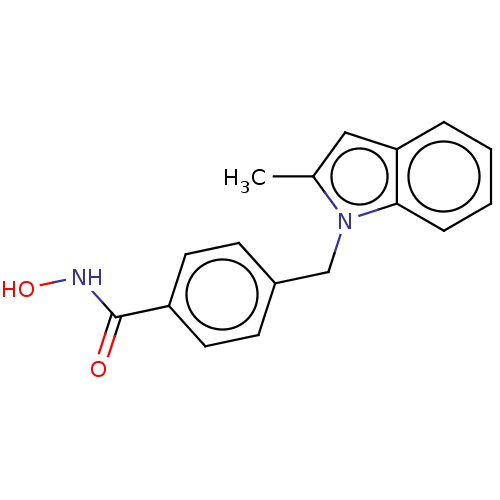
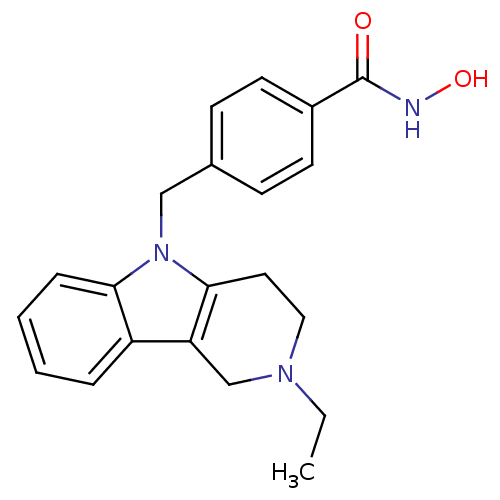
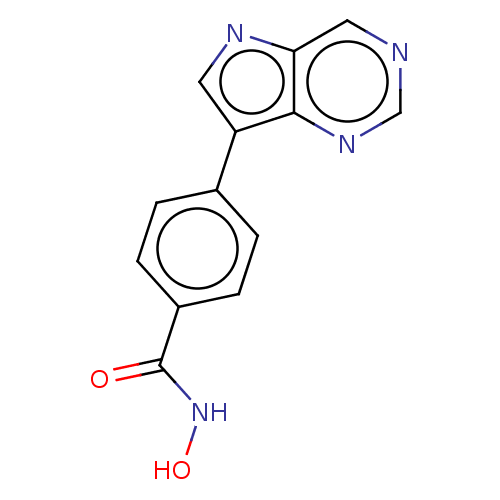
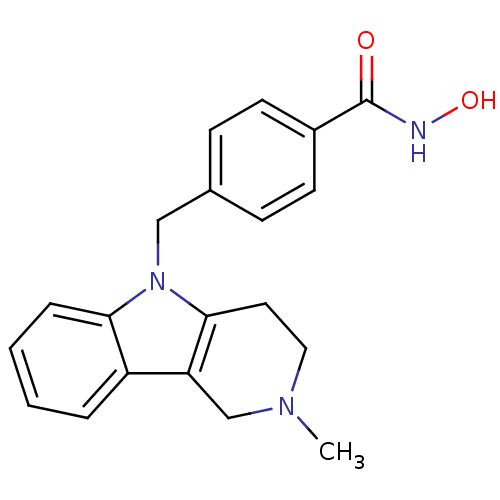
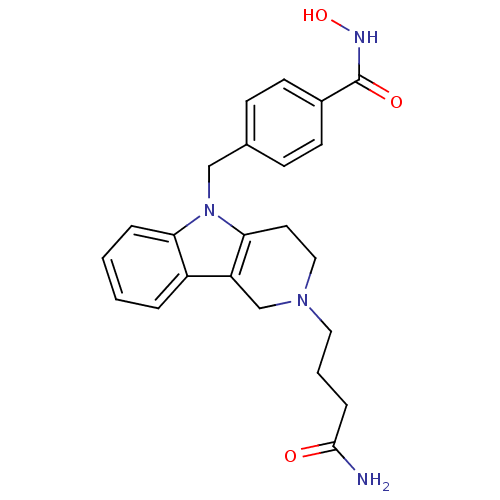
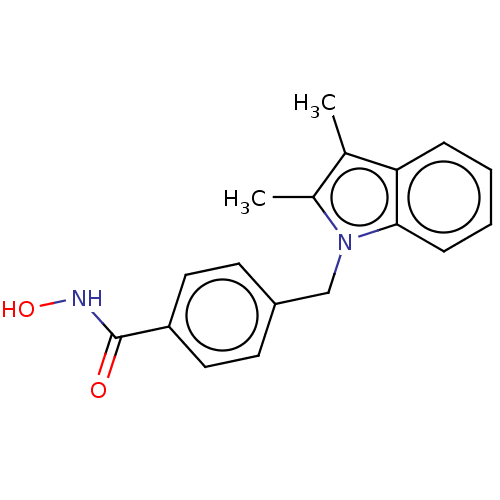
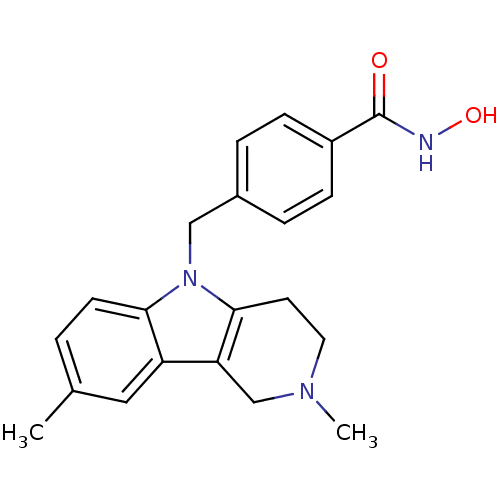
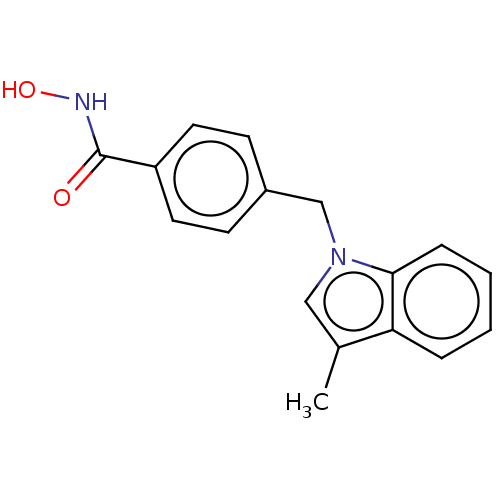
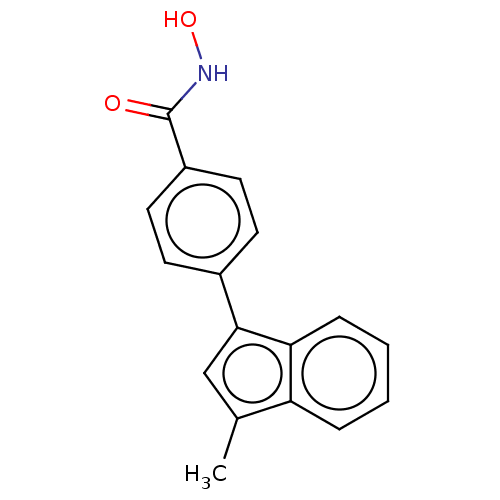
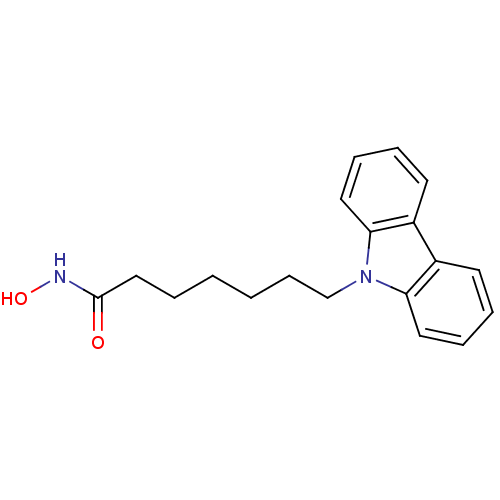
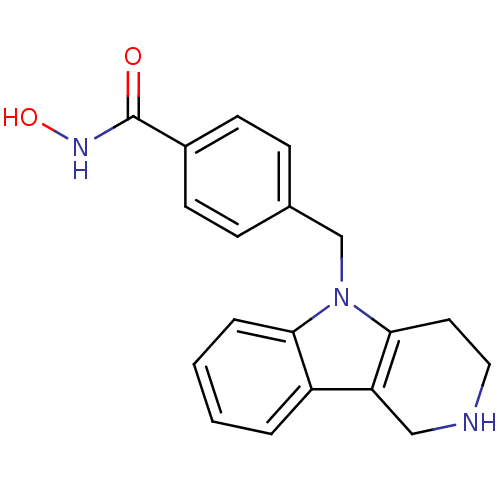
Affinity DataKi: 0.800nM IC50: 4nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
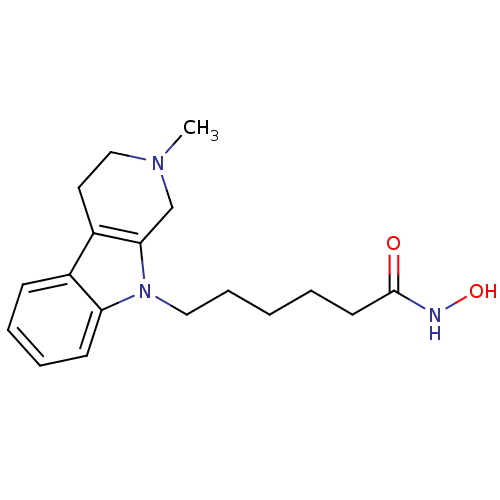
Affinity DataKi: 1.30nM IC50: 5nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
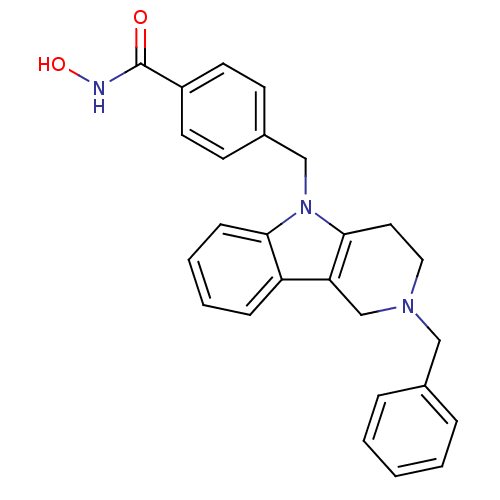
Affinity DataKi: 3nM IC50: 9nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
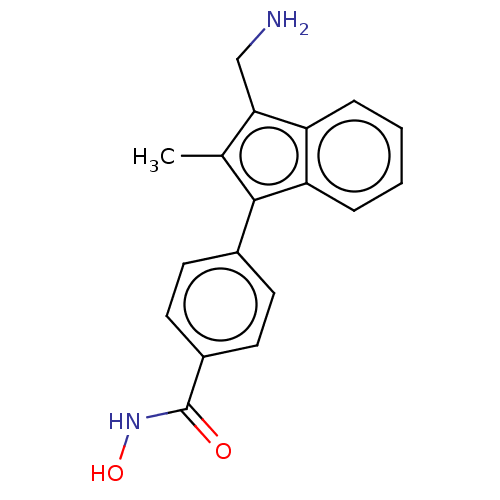
Affinity DataKi: 11nM IC50: 30nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 17nM IC50: 42nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 23nM IC50: 83nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 55nM IC50: 119nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 110nM IC50: 230nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 120nM IC50: 250nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 120nM IC50: 260nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: 550nM IC50: 1.10E+3nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
TargetProtein-S-isoprenylcysteine O-methyltransferase(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Competitive inhibition of human isoprenylcysteine carboxyl methyltransferase expressed in yeast His10myc3N-Icmt membranes using increasing AFC substr...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nM IC50: 3.00E+3nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
TargetProtein-S-isoprenylcysteine O-methyltransferase(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Non-competitive inhibition of human isoprenylcysteine carboxyl methyltransferase expressed in yeast His10myc3N-Icmt membranes with increasing [14C]-S...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM IC50: >2.00E+4nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: >3.75E+4nM IC50: >7.50E+4nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nM IC50: >1.00E+5nMAssay Description:In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.459nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.582nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.704nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.799nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.872nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.972nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 1.44nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.46nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 1.46nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 2.25nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.49nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 2.94nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 2.94nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 2.96nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.99nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.05nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.06nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.48nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 3.48nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 4.06nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.08nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.11nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 5.11nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 8.0 T: 2°CAssay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 6.56nMAssay Description:Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrsMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)